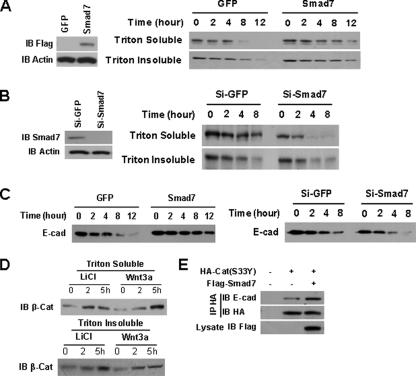
FIGURE 7.
Smad7 stabilizes E-cadherin-β-catenin complex and increases β-catenin binding to E-cadherin. A, Smad7 increases the stability of β-catenin (β-Cat). NMuMG cells expressing GFP or Smad7 were treated with 50 μg/ml cycloheximide for the indicated lengths of time, and the cells were extracted with Triton X-100. Then β-catenin in Triton X-100-soluble or -insoluble fractions was detected by Western blotting. Equal amounts of protein from each group were loaded for SDS-PAGE. B, knockdown of Smad7 in NMuMG cells decreases the stability of β-catenin. NMuMG cells were transfected with Si-GFP or Si-Smad7 siRNA, and the stability of β-catenin was analyzed as described in A. C, the stability of E-cadherin (E-cad) is regulated by Smad7. NMuMG cells were infected with GFP or Smad7, as well as were transfected with siRNAs the same as B, and then treated with 50 μg/ml cycloheximide for the indicated lengths of time. Whole cell lysates were detected by Western blotting. Equal amounts of protein from each group were loaded for SDS-PAGE. D, Western blotting analysis of β-catenin in Triton X-100-soluble and -insoluble extraction fractions prepared from T47D cells treated with LiCl (30 mm/liter) or Wnt3a for 2 or 5 h. Equal amounts of protein from each group were loaded for SDS-PAGE. E, immunoprecipitation analysis of the effects of Smad7 on the association of β-catenin (S33Y) with E-cadherin. FLAG-Smad7 plasmids were co-transfected with HA-β-catenin (S33Y) into 293 cells. The cell lysates were subjected to immunoprecipitation with anti-HA antibody. The endogenous E-cadherin in the precipitated complexes was blotted as indicated. IB, immunoblot. Si, small interfering RNA.