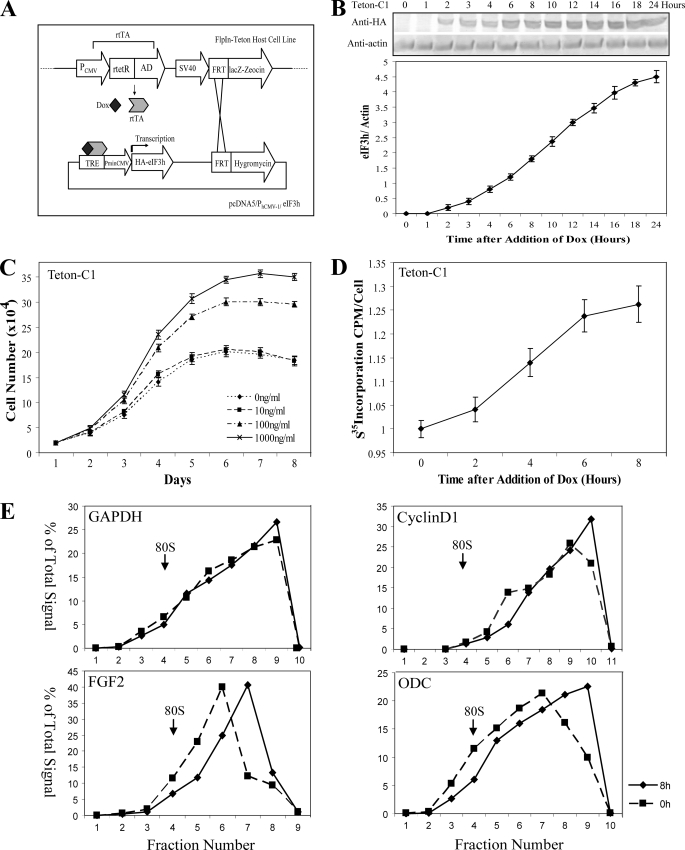
FIGURE 5.
Effects of rapid induction of eIF3h expression in NIH-3T3 cells. A, schematic for the construction of an inducible FlpIn-Teton-eIF3h expression system. The FlpIn-Teton-3T3 host cells contain a Flp recombination target (FRT) site and express the reversible tet-responsive transcriptional activator (rtTA). The inducible eIF3h expression cassette (or the empty vector) is integrated into the FRT site through a site-specific recombination event. After addition of doxycycline (Dox), rtTA binds to the upstream tet-responsive element (40) and enables the minimal cytomegalovirus promoter (PminCMV) to express HA-tagged eIF3h. B, time course of expression of HA-eIF3h in Teton-C1 cells induced with 1 μg/ml Dox. Cell lysates were prepared at the indicated times and analyzed by immunoblotting with anti-HA and anti-actin antibodies (upper panel). The HA-eIF3h/actin ratio at each time point was calculated and graphed below. C, growth curves of Teton-C1 cells maintained in 0, 10, 100, and 1000 ng/ml of Dox were determined as described in the legend to Fig. 3B. D, rate of [35S]methionine incorporation into protein in Teton-C1 cells measured at the indicated times after treatment with 1 μg/ml Dox. Incorporation at 0 Dox is 1.22 cpm per cell, normalized to 1. The data in B-D are means ± S.D. of three independent experiments, each done in triplicate. E, Teton-C1 cells treated0h(squares) and 8 h(diamonds) with 1 μg/ml Dox were lysed and subjected to sucrose gradient centrifugation as described previously (7). Ten gradient fractions were collected, and total RNA was isolated from each fraction and analyzed by Taqman real time RT-PCR using specific primer and probe sets as described previously (7). Quantitation of mRNA in each gradient fraction is reported as the percentage of the total amount present in all fractions. Sedimentation is from left to right; the sedimentation position of 80 S ribosomes is indicated by an arrow.