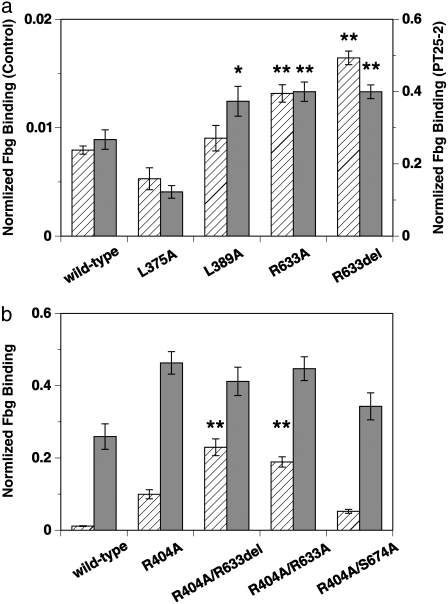
FIGURE 7.
Average amount of FITC-labeled Fbg bound to CHO cells expressing wild-type or mutated αIIbβ3 obtained from six separate experiments. The amount of Fbg in the presence of control antibody (hatched bars) and PT25-2 (shaded bars) is shown separately in arbitrary units with error bars. (a) Results for mutations at the residues involved in the Arg633-mediated interactions are compared with those for the wild-type. Amino acid residues Leu375, Leu389, and Arg633 in the β3-chain were either mutated to Ala (L375A, L389A, and R633A) or deleted (R633del). The amount of Fbg in the presence of the control antibody and PT25-2 is shown in the left and right axes, respectively. Mutants that showed a statistically significant increase in binding from the wild-type are indicated by asterisks: *p < 0.05; **p < 0.01. (b) Results for single mutation of Arg404 in the β3-chain (R404A) and double mutations of Arg404 in combination with either Arg633 (R404A/R633A, R404A/R633del) or Ser674 (R404A/S674A) are shown and compared with those for the wild-type. Mutants that showed significant increase in binding from the wild-type are indicated by asterisks: **p < 0.01.