Abstract
The fathead minnow is a useful species for evaluating the toxicity of wastewater effluents. While this fish is widely used for “survival” studies of metal toxicity, little or no work has been done on the tissue distribution of metals in fathead minnows. To determine the distribution of tissue lead, aquarium studies were conducted for several weeks with fish maintained in soft synthetic freshwater. Lead II nitrate was added to 3 aquaria attaining concentrations of 20 - 30 ppb (aquarium B); 100 - 140 ppb (aquarium C); and roughly 200 ppb (aquarium D). Results were compared to controls (aquarium A). During the initial week, the majority of aquarium D fish died, whereas few deaths occurred in the other groups. Lead accumulation was dose- and tissue-dependent, with highest uptake by the gills. Gill concentrations of aquarium D fish averaged about four-fold higher than in skeleton or skin and muscle. In vitro, lead (2.5 to 25 ppm) caused dose-dependent reductions in the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) in gills incubated in physiological buffer. These findings demonstrate that fathead minnow gills bind and accumulate waterborne lead rapidly and preferentially, and raise the possibility that gill lipid peroxidation contributes to lead toxicity at low water hardness.
Introduction
The fathead minnow, Pimephales promelas, is a freshwater fish widely used for biomonitoring of wastewater discharges; i.e., Whole Effluent Testing (WET), to meet the objectives of the National Pollutant Discharge Elimination System (NPDES) Permits Program. The USEPA has developed guidelines for toxicity testing which rely on this and other species. In vivo and in vitro studies with fathead minnows (1) have advanced our understanding of the effects of endocrine-disrupting chemicals. Weber has reported (2) that chronic (4 weeks) exposure to waterborne lead concentrations of 500 ppb may alter reproductive behavior in adult fathead minnows. The same laboratory (3) also observed effects on feeding behaviour and whole brain neurotransmitters in juvenile fathead minnows at lead concentrations of 500 ppb - 1 ppm. Tissue lead distribution was not examined in these studies. While there are many practical advantages to a bioassay with fathead minnows, including a very extensive database for organic and inorganic compounds, limited information is available on the toxicokinetics of lead or other heavy metals in the fathead minnow. On the other hand, a good deal of information is now available on the accumulation of mercury (4,5), zinc (6), cadmium (6) and lead (7) in muscle and other body compartments of commercially important food and game fish. Very often, partitioning of metals among tissues has been the subject of field studies where investigators have considered the uptake of metals into fish exposed to complex mixtures of pollutants (8-10).
The fathead minnow is not a species of choice for tissue distribution studies due to its small size (adults over 5 grams are rare). Moreover, studies of the effect of waterborne divalent lead are particularly challenging, owing to the difficulty in maintaining stable aquarium lead concentrations (e.g.; insolubility of lead salts, redox reactions, binding to particulates). The primary aim of this investigation was to determine the tissue distribution of lead after chronic exposure (up to 25 days) of adult fathead minnows to waterborne divalent lead (Pb++). In contrast to previous aquarium studies, we monitored aquarium lead concentration frequently and adjusted lead concentration to different “steady state” or “plateau” levels; i.e., low, moderate and high doses, by replacing any loss of waterborne metal with additional lead nitrate. These aquarium lead concentrations were expected to be sublethal based on previous work with rainbow trout (11) and fathead minnows (2, 12) studied under laboratory conditions. In order to mitigate the problem of the low solubility of most lead salts, we exposed fathead minnows to lead nitrate in a special synthetic freshwater (SFW) formulation prepared without sulfate and carbonate/bicarbonate anions. Conducting aquarium studies with very soft water is a useful approach to deal with the problem of precipitation of lead salts. On the other hand, this approach can be problematical in that biological filtration systems may not be as effective when pH <6.5 (13), and comparison of results with those obtained with natural water may not be as straightforward. We elected to perform our lead exposure experiments using aquaria filled with very soft synthetic freshwater without carbonate or sulfate anions. Chloride was substituted for these anions. The solubility of PbCl2 is about 100-fold greater that Pb(SO4)2 at 25 °C (Ksp = 1.6 × 10-5 vs. 1.3 × 10-8). However, after the initial week of exposure, it became clear that the high lead dose was not sublethal. In view of reports (14-16) that lead can cause oxidative stress to cell membranes of vertebrate species, and to shed light upon the possible mechanism for the observed toxicity, in vitro experiments were also carried out to evaluate possible lead-induced oxidative injury to gill membranes.
Experimental Section
Fish Holding Conditions
Adult fathead minnows (0.90 g - 4.20 g, 23 males, 25 females) were obtained from Aquatic Research Organisms (ARO)(Hampton, NH). All procedures involving the use of animals were reviewed and approved by the UMDNJ School of Osteopathic Medicine Animal Care and Use Committee. Fathead minnows were initially kept in a 125 gal holding aquarium (closed system) containing hard SFW (prepared according to USEPA document 600/4-90/027F; hardness 160 - 180 mg/L as CaCO3, pH 7.8, with 1 % Instant Ocean; salinity = 0.35 ppt) under simulated natural photoperiod (12L:12D) and constant temperature (25 °C). The composition (mg/L) of the hard SFW used in this holding aquarium was as follows: NaHCO3 192.0, CaSO4 dihydrate 120.0, MgSO4 120.0, and KCl 8.0. The aquarium was equipped with biological filtration; water changes of 10 - 50% were made as needed. Animals were fed twice daily with TetraminR flake food. The SFW formulations were routinely prepared with reverse osmosis water passed through a commercial deionizing system. The electrical resistance of the reverse osmosis-deionized water (dH2O) was 18-megaohm/cm. Levels of nitrite and ammonia were analyzed with TetraR Kits and pH was determined with a Fisher AccumetR pH meter.
Chronic Lead Exposure Protocol
The studies of chronic lead exposure were carried out using four 12 gal plastic aquaria (EclipseR, Marineland, Moorpark, CA), with one control, lead-free aquarium (aquarium A) and three experimental aquaria in which concentrations of lead nitrate were adjusted to different dose levels. Doses were selected to elucidate the effect of a one order of magnitude difference in tank lead concentration on tissue lead accumulation. Tank lead concentration was monitored at frequent intervals using Hach LeadTrakR Kits (see below). Target lead concentrations were: a low dose level of approximately 25 ppb, aquarium B; a moderate dose of lead of about 100 ppb, aquarium C; and a high dose of lead approaching 250 ppb, aquarium D. The total measured volume of a filled 12 gal EclipseR aquarium was 43.920 L.
In pilot experiments without fish and with various filter systems, we were unable to maintain stable aquarium Pb concentrations using hard SFW. For subsequent aquarium studies with fish, we used a very soft water formulation of synthetic freshwater (very soft SFW), identical to that described in EPA document # 600/4-90/027F, except for replacement of sulfate and carbonate ions by chloride ions. The tissue distribution studies were carried out in two runs. In the first run, a single aquarium was used (aquarium C above) to assess the feasibility of maintaining a given aquarium concentration and to ascertain if a concentration of about 100 ppb was well tolerated. After completing this feasibility study, a second run was performed to examine tissue distribution at other concentration levels. In this second run, two experimental aquaria (B and D) and a control aquarium (A) were set up and studied in parallel fashion.
Throughout the entire study, precautions were taken to eliminate splashing or leakage of aquarium water. The aquaria were placed on plastic backed absorbent paper and each aquarium was enclosed in a large plastic basin (RubbermaidR). These basins were kept in the UMDNJ - SOM animal facility on sturdy lab carts with high borders. Tank lids were tight-fitting and water evaporation was minimal (<3 % over a one month period).
Lead was introduced by the following procedure: aquaria (12 gal) were filled with sulfate-free, carbonate-free, very soft SFW and the filter trays were loaded with sponge filters and BiokaskadeR media. After approximately 2 weeks in the holding aquarium, 2 or 3 fish were transferred into each 12 gal aquarium. The filter unit in the aquarium cover was turned on, and time was allowed for bacteria to grow on the filtration media. Initially, we carried out 50% water changes every few days; later, once ammonia levels fell below 0.25 mg/L, 10-20% renewals were made. Subsequently, another pair of fish was transferred from the holding tank into each 12 gal aquarium. The process was repeated until each aquarium housed 7 - 9 fish. On day zero of the study, a small aliquot (0.26 to 1.00 mL) of lead nitrate stock (either 0.78, 7.8 or 10 mg/mL as lead in dH2O) was pipetted into aquaria B, C and D. A volume of dH2O equivalent to that used for the high dose aquarium was pipetted into the control aquarium. This dosing procedure was repeated at intervals of 2 - 3 days, depending on the results of the aquarium water assays (see below). Levels of nitrite and ammonia, as well as pH, were routinely evaluated in all aquaria as above.
Tissue Sampling
The following animal handling and surgical procedures were carried out using disposable gloves, plastic backed absorbent bench covers and protective work clothing. Fish (n=32, 17 females, 15 males) were removed from control and experimental tanks at the indicated times and euthanatized (pithing followed by decapitation). The exterior of the fish was quickly rinsed with dH2O and tissues were isolated by blunt dissection. After removal of the gills with fine scissors, a ventral abdominal incision was made, exposing the intestines and other viscera. The intestines and all soft tissue within the abdominal cavity were collected; these tissues are referred to as the “viscera” sample. Muscle and adherent skin on both sides were separated from the spine using a scalpel; these 2 sides (“filets”) combined represent the skin and muscle sample. The skeleton sample consisted of the vertebral column with attached ribs but did not include fins or the branchiocranium. All tissue samples were rinsed with dH2O, blotted on filter paper and placed into tared glass scintillation vials for weighing. Tissues were also collected from all fish found dead in the aquarium, typically at the morning feeding. Any fish found dead in a tank was placed in a plastic bag, refrigerated (4 °C for <24 h) and dissected as described. After weighing, the four samples from each fish were frozen at -80 °C.
For measurement of tissue lead, samples were digested in 3:1 nitric/perchloric acid and analyzed by flameless atomic absorption spectrophotometry (AAS) using a Perkin Elmer Z5100. Ashing (digestion with nitric/perchloric acids; 3:1 v/v) was done in open beakers on a hot plate with a surface temperature of 140 - 175 °C for 2 - 3 hours until almost all acid had evaporated. Thus, the maximum ashing temperature is the boiling point of the acid mixture. All tissue material, including lipids, was thoroughly digested by this procedure using the above strongly oxidizing acid mixture. To prevent tissue contamination with metals, all glassware was washed with a laboratory soap solution (Contrad 70), then rinsed with tap and finally distilled water. This was followed by soaking of the beakers in 20% nitric acid for 24 h followed by thorough rinsing with distilled/deionized water. Analysis of two or more blanks with each set of tissues verified that contamination from metals in the glassware or acids used was not detectable or minimal compared to the much higher metal concentrations found in the fish tissues. Non-zero blank concentrations were subtracted from the gross tissue lead concentrations to calculate the net tissue concentration. Tissue concentrations of lead are expressed as μg Pb per gram dry weight (ppm).
Analysis of Lead Concentration in Aquarium Water
Aquarium lead concentration was monitored periodically using a Hach DR/2010 spectrophotometer and Hach LeadTrakR - fast column Kits. Water samples (40 - 60 mL from aquaria B, C and D; 100 - 120 mL from aquarium A) were collected at intervals of 2 - 3 days with plastic syringes. Volume was not replaced. The Hach procedures for lead analysis (fast column extraction method) are based on assay with dithizone after nitric acid extraction of water samples. The limit of detection of lead in water using the LeadTrakR Kit procedure is 1 ppb according to the manufacturer. The limit of detection for the AAS method is <0.2 ppb. Values for tank lead concentration as determined by Hach LeadTrakR assay were checked by flameless AAS.
In Vitro Studies of Gill Oxidative Injury
Additional experiments were carried out to determine whether divalent lead is capable of causing oxidative stress to gill epithelium. Lead-induced oxidative stress was investigated in isolated fathead minnow gills by simultaneous measurement of tissue levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) using a commercially available kit (GSH/GSSG-412R Assay System, Oxis ResearchR, Portland, OR). For this study, another group of adult fish (n=16, 1.33 - 3.11 g; 8 males, 8 females) from the holding tank were euthanized as above. Gills were excised rapidly, taking care to maintain the ventral connection between arches. A complete set of gills from each fish was transferred as a single piece into a plastic flask (Nalge) containing 10 mL of ice-cold modified Cortland’s saline (composition in g/L: NaCl 9.36,, KCl 0.194, MgSO4 heptahydrate 0.2293, NaHCO3 1.495, NaH2PO4 monohydrate 0.414, CaCl2 dihydrate 0.228, and d-glucose 1.008). Gills from 2 - 6 fish were incubated concurrently in a shaker water bath at room temperature using published methods (17). An aliquot (100 μL) of lead nitrate stock solution in dH2O (12 - 120 μM, final concentration) was then added, and the flasks were incubated (low shaker speed) under an atmosphere of 95% O2/5% CO2. After 60 min, the tissue was removed, blotted on filter paper, and separated into right and left sets. Each side was weighed and homogenized separately to determine both GSH and GSSG in gills from the same fish. Levels of GSH and GSSG were measured using a BioxytechR GSH/GSSG-412TM Kit (Oxis Research, Portland, OR). This recycling assay, adapted from the method of Tietze (18), depends on glutathione reductase-catalyzed transfer of reducing equivalents from NADPH to GSSG. Reactions were followed with Ellman’s reagent by measuring the rate of change in absorbance at 412 nm using a HP8452A diode array spectrometer with ChemStationR software.
Statistical Analyses
The results of the tissue lead determinations and the in vitro data (GSH/GSSG) were analyzed using GraphPad PrizmR (Version 4) software (GraphPad Software, Inc., San Diego, CA). For the aquarium study, Two-way ANOVA was used to evaluate possible effects of tissue and dose. This analysis was limited to the 28 animals where measurements were made on all 4 tissues (results depicted in Fig. 2; n=7 for controls; n = 5-8 for experimental groups). The null hypothesis was that the lead concentration in the water had no effect on tissue lead in the different samples. For any given tissue sample, error exists for the individual fish in each aquarium due to many independent variables affecting the toxicokinetics. There will also be an error term for the way each aquarium is prepared which contributes to the total variability; accordingly, two factors can be used to partition the variability (personal communication, Dr. S. C. Gad). Multiple group comparisons were carried out using the Bonferroni correction. The critical Bonferroni value was t0.0055 as 9 comparisons were of interest (aquarium A controls not included, Fig. 2). One-way ANOVA was used to test for a possible dose effect of in vitro lead exposure on GSH/GSSG ratio (n=4 whole gill preparations/dose). Data are reported as mean ± SEM. A p-value ≤ 0.05 was considered statistically significant.
FIGURE 2.

Mean levels of lead (μg/g dry weight) in major tissue compartments of control and lead nitrate-exposed fathead minnows. Analysis of these data by Two-way ANOVA revealed a highly significant effect of dose (p ≤ 0.0003) as well as a significant (p ≤ 0.0096) effect of tissue. Results for Tank A controls sacrificed on days 7 or 21 were pooled. Note the ten-fold difference in scale used for controls. * p ≤ 0.05, ** p ≤ 0.01 Significantly different when compared to the mean concentration in the gills at the same dose.
Results
Prior to addition of lead nitrate, the concentration of lead in all aquaria was at or near the limit of detection (1 ppb, Hach LeadTrakR method). During the set up period and on study day zero, none of the test animals (7 - 9/aquarium) exhibited adverse health effects such as circling behavior or disinterest in food. Following the repeated addition of lead nitrate solution, aquarium lead levels progressively rose (Fig. 1) in the experimental aquaria B and C, tending to plateau after 2 weeks at levels of about 25 and 100 ppb, respectively. By contrast, the lead concentration of aquarium D increased abruptly and exhibited wide variation, ranging from 114 to 231 ppb on days 5 - 7. Over the same period, high mortality was encountered; e.g., 3 of the aquarium D fish died on study day 6. The two fish that survived in aquarium D on day 8 were euthanized, along with 3 control fish. These controls were selected at random, to check on baseline lead levels in naïve fish. The lead concentration in water from the control aquarium (A) remained near or below the limit of detection (Hach LeadTrakR method) throughout the study (Fig. 1).
FIGURE 1.

Lead concentrations in experimental and control aquaria during chronic study of tissue lead distribution in adult fathead minnows. Aliquots of lead nitrate stock solution (0.26 - 1.00 mL in water) were repeatedly added to tanks B, C and D to attain “low”(B), “moderate”(C) and “high” (D) doses. The majority of aquarium D fish died during the initial week. Lead concentration in the control aquarium (A) remained near the limit of detection (1 ppb, HachLeadTrakR method) throughout the study. For aquarium B, ten additions were made, using either 0.78 mg/mL as Pb (the initial 5 additions ranged from 0.51 - 0.78 mg Pb)) or 7.8 mg/mL as Pb (the subsequent 5 additions ranged from 2.03 - 4.5 mg Pb) of Pb(NO3)2 stock solution in dH2O. The cumulative amounts of Pb added after 7 and 21 days were 3.36 mg and 18.89 mg, respectively. For aquarium C, seven additions were made, using 10 mg/mL as Pb of Pb(NO3)2 stock solution. The amount of lead per addition ranged from 4.4 mg to 8.78 mg. The cumulative amounts of Pb added after 7 and 26 days were 26.33 and 43.93 mg as Pb. For aquarium D, five additions were made, using 7.8 mg/mL as Pb of Pb(NO3)2 stock solution. The amount of lead per addition ranged from 5.1 mg to 7.8 mg. The cumulative amount of Pb added after 7 days was 33.6 mg as Pb.
The concentration of lead in various tissues of fathead minnows after chronic exposure to lead nitrate in aquarium water is shown in Table 1. Gills accumulated high concentrations of Pb at all dose levels. The lead concentration in the soft visceral tissues exhibited wide variation, especially in animals from aquaria C and D. The lead concentration in the viscera of two fish were extremely high (615.78 ppm in one aquarium C fish, and 726.59 ppm in one aquarium D fish). At low and moderate concentrations of lead nitrate in aquarium water, the accumulation of Pb was clearly dose-dependent in the 4 tissues examined (Table 1, Fig. 2). The accumulation of skeletal lead was particularly marked in aquarium C animals (Table 1, Fig. 2). Mean values for total tissue lead (ng) were calculated for aquaria C and D fish. These data indicated the following rank order (from highest to lowest mean Pb in total biomass): for aquarium C fish; viscera (6086 ± 3283) > skin and muscle (4992 ± 713) > skeleton (4395 ± 514) > gills (2165 ± 214); and for aquarium D fish, skin and muscle (4705 ± 1448) > viscera (1943 ± 1504) > gills (1827 ± 518) > skeleton (589 ±104 ).
Table 1. Mean levels (± SE, μg/g dry weight) of lead in different tissues of lead nitrate-exposed and control fathead minnows.
Fish in experimental aquaria D, B and C were sacrificed after 7, 21 or 25 days of lead nitrate exposure, respectively. Control fish (aquarium A), studied concurrently with fish in aquaria B and D, were euthanized after 7 or 21 days. Values from fish found dead at the morning feeding are included in this table. On the day lead nitrate was first added to the aquaria (study day 0), the number of fish housed in aquaria A, B, C and D were 8, 7, 9 and 8, respectively
| Tissue μg/g dry wt. | Control (Tank A) | Low Dose (Tank B) | Medium Dose (Tank C) | High Dose (Tank D) |
|---|---|---|---|---|
| Gills | 0.141 ± .10 | 54.040 ± 5.39 | 197.292 ± 26.46 | 191.892 ± 67.33 |
| Skin/Muscle | 0.448 ± .09 | 15.303 ± 3.26 | 32.02 ± 6.58 | 40.576 ± 14.33 |
| Viscera | 0.411 ± .17 | 12.764 ± 3.00 | 181.719 ± 86.30 | 91.225 ± 75.01 |
| Skeletal | 0.265 ± .07 | 17.351 ± 3.42 | 81.559 ± 12.46 | 16.619 ± 3.55 |
Note: One aquarium A fish was found dead on study day 3. Two other aquarium A animals died on days 13 and 14. Three control fish were euthanatized on day 8. Five aquarium B fish died (2 on days 14 and 15; 3 on day 19). One aquarium C fish was found dead at the end of the study (day 25). Six aquarium D fish were found dead (1 on day 1, 2 on day 4 and 3 on day 6).
Two-way ANOVA indicated a significant dose effect (p=0.0003) as well as a significant tissue effect (p=0.0096). No interaction was detected between tissue and dose (p=0.2713). When mean lead concentrations in the various tissues of animals in aquaria B, C and D (Fig. 2) were compared by the Bonferroni method, significantly higher lead concentrations were found in the gills of aquaria D fish than in either the skeleton or the skin/muscle sample. The gills of aquarium C fish also accumulated significantly (p<0.05) more lead as compared to skin and muscle.
The high mortality (80%) seen with the aquarium D animals after one week of lead nitrate exposure contrasted sharply with the lack of adverse health effects seen at the lower dose levels (Fig. 3). In the other groups, only one fish did not survive the first week; i.e., one of the 8 aquarium A controls died on study day 3 (12.5% mortality). After 12 days, a number of deaths were noted in the aquarium B group, such that only about one-third of aquarium B fish survived for the duration of the study. No deaths occurred in aquarium C after 25 days of lead nitrate exposure.
FIGURE 3.
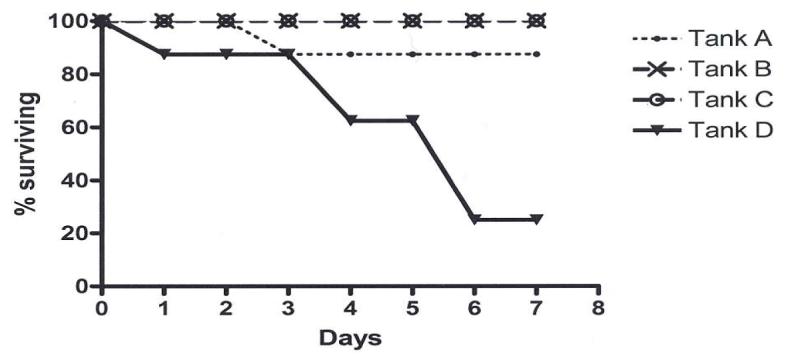
Survival rates of fathead minnows during the initial week of exposure to different aquarium concentrations of lead nitrate (aquarium A: unexposed controls; aquarium B: 20 - 30 ppb; aquarium C: 100 - 140 ppb; aquarium D: 150 - 250 ppb). Actual aquarium concentrations varied over time as in Figure 1.
The pH of the aquarium water was of concern since the carbonate-free, sulfate-free very soft SFW used in all aquaria would have a reduced buffering capacity relative to standard SFW. As is apparent from Table 2, pH was quite low (5.3 - 6.0) in all aquaria at the start of the study. While the pH in the individual aquaria was stable, changing less than 0.5 pH unit over 3 weeks of monitoring, the pH of aquarium C water was distinctly elevated relative to the other aquaria. Nitrite and ammonia levels as determined with Tetra KitsR were identical in Tanks A, B and D. Ammonia concentration (mg/L) was 1.5 on day 7 and 3.0 on days 16 and 20. Nitrite concentrations on the same days were less than 0.3 mg/L. These measurements were not available for Tank C except on the last day of the study (day 25) when an ammonia concentration of 5.0 mg/L was observed.
Table 2. pH of Aquarium Water During Chronic Treatment with Lead Nitrate.
| Study Day: -1 | TANK: A | TANK: B | TANK: C | TANK: D |
|---|---|---|---|---|
| 5.7 | 5.3 | 6.0 | 5.3 | |
| 7 | 5.2 | 5.6 | 6.5 | 5.4 |
| 16 | 5.6 | 5.6 | 6.4 | - |
| 20 | 5.3 | 5.5 | 6.3 | - |
| 25 | - | - | 5.6 | - |
Measurements of lead concentration were made in a small set of samples using both the Hach method and AAS. Respective values for aquarium lead concentration (ppb) measured with the Hach method and AAS were < 1 vs. ND for aquarium A on day 21; 38 vs 49.45 for aquarium B on day 21; 114 vs 125.4 for aquarium C on day 16; and 231 ppb vs. 237.45 for aquarium D on day 7. The laboratory dH2O routinely used for dilution of aquarium samples was <1 ppb by the Hach method and 0.6ppb as determined by AAS. It is evident that the two analytical methods were in close agreement (r2 = 0.98, p< .0001, n=7 data pairs). A lead nitrate stock solution used for the in vitro studies was prepared as 250 ppm in dH20; the concentration as determined by AAS was 282.8 ppb.
Dose-dependent oxidant stress was observed when isolated fathead minnow gills were incubated with 12, 60 or 120 μM lead nitrate (Fig. 4). These concentrations are equivalent to a final metal concentration in each flask of 2.5, 12.5 and 25 ppm, respectively. When one-way ANOVA was used to analyze the effect of lead on the GSH/GSSG ratio, the dose effect was significant at the 5% level.
FIGURE 4.
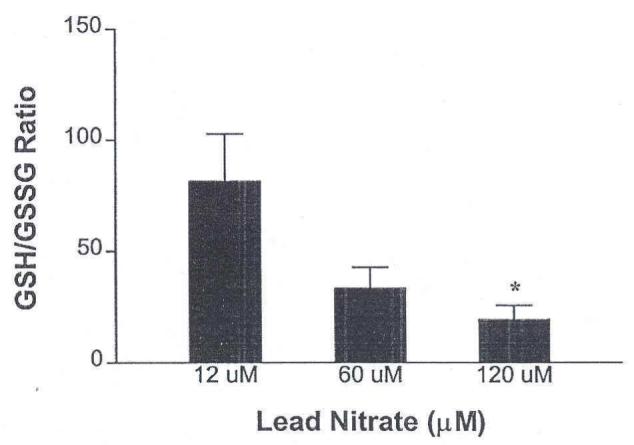
Lead-induced oxidative stress in isolated fathead minnow gills. Whole gill preparations from naïve adult fish were incubated with lead nitrate for 60 min according to published methods (17). n = 4 fish/lead concentration. Average GSH/GSSG values for the vehicle control group (n=4) averaged 83.02 ± 20.55 * p<0.05 relative to vehicle controls as determined by ANOVA with modified t-statistics (Bonferroni method).
Discussion
The results of the present study demonstrate that waterborne lead accumulates selectively in the gills of the fathead minnow, relative to other tissues and organ systems. We are not aware of any previous studies of the tissue distribution of lead in this species. All animals were laboratory-raised, with only trace levels of lead in tank effluents or supply water (< 3 ppb according to ARO water analysis reports). The lead content of all tissues of control fish (aquarium A) averaged less than 1 ppm, and, unlike the situation in the experimental groups, the lowest mean lead concentration was found in the gills, compared to viscera, skin and muscle and skeleton. Our finding that the gills accumulated the highest lead concentration is in agreement with the results of a very recent report by Grosell et al. (19) who examined tissue lead distribution in larval fathead minnows.
Recently, there has been heightened research interest in the role of the gills of teleost fish as a marker for contaminant effects. Malins et al. (20) provided evidence for changes in gill DNA in bottom fish from PCB-contaminated sediments and developed a gill “DNA damage index”. Substantial progress has also been made in modeling factors involved in metal uptake by gill tissue as it relates to acute toxicity (21,22). However, few aquarium studies have been carried out to clarify the concentration-dependence of uptake of lead by the fish gill. Over the last decade, scattered reports have appeared indicating that lead may preferentially accumulate in the gills of freshwater teleost fish.
In an early preliminary report, Preslan and coworkers (23) evaluated tissue lead levels in sunfish (Lepomis) after exposure to lead nitrate at concentrations comparable to our “moderate” dose (aquarium C). After 100 days of exposure to reported concentrations of lead nitrate of 50 or 100 ppb, these investigators found that the gills accumulated considerably higher concentrations of lead, compared to skin, viscera, muscle, and skeleton. More recently, Ay et al. (7) reported that the gills of Tilapia zilli accumulated much higher concentrations of lead at all dose levels of lead nitrate (0.5 mg/L to 4 mg/L) compared to muscle and liver. Although aquarium lead concentration was not analyzed in the latter study, the aquarium water was changed every 2 days to mimimize the fall in lead level.
We found that the abdominal viscera of fathead minnows accumulated substantial lead especially at moderate and high doses. Lead accumulation in the “viscera” sample includes uptake by intestine, kidney and liver. Avid uptake of Pb++ by these organs is consistent with observations of Thomas and Juedes (24) in a study with the saltwater fish Micropogonias undulates. The latter group found the greatest accumulation of 210Pb in the intestine after administering radiolabeled lead nitrate in the diet along with unlabeled lead nitrate for prolonged periods (up to six weeks). Accumulation of 210Pb in gill tissue was not examined.
Other heavy metals seem to share lead’s ability to bind or penetrate into gill tissue. Work with freshwater fish exposed to mercuric ion or methylmercury suggests that relatively high levels of mercury accumulate in the gills (25). Similarly, gill concentrations of cadmium were especially high when juvenile rainbow trout were exposed to cadmium nitrate in aquarium water for 30 days, with tissue concentrations in the order kidney>gill>liver> whole body>carcass (6).
The extent that Pb++, added to aquarium water, enters the blood of the fathead minnow via the intestine is an open question. We speculate that a large fraction of the total body burden of lead resulted from absorption through the GI tract. Significant amounts might enter due to ingestion of lead absorbed onto food particles, which could explain the extremely high values, >600 μg/g dry weight, measured in the viscera samples from 2 fish. Blood lead in fish and man is carried mainly within erythrocytes (12, 26), and the high concentrations of gill lead may reflect uptake from blood passing through the gills.
Slow movement from blood to bone would be expected. With our experimental design, the aquarium C fish were exposed to aquarium lead concentration roughly one-half that of aquarium D, but the period of exposure was considerably longer. The five-fold greater lead concentration in the skeleton of these animals (Table 1), relative to group D, probably reflects the longer exposure period and gradual incorporation and sequestration in bone (27). In this regard, it is noteworthy that in the work with larval fathead minnows cited above (19), the skeleton accumulated the largest mass of lead, although gill concentrations were the highest. Due to differences in experimental design (e.g., composition of aquarium water, duration of exposure, flow-through vs. static, adult vs. larval fish), it is difficult to draw comparisons, but with our experimental design, the mass of lead in the skeleton was less than the total amounts in viscera or skin and muscle.
More than half the fish in aquarium D died within one week after exposure to lead at levels of 100 - 200 ppb. The deaths seen at this dose contrasted with the lack of adverse effects noted in the other aquaria where one week survival rates were ≥ 87.5% despite the very soft aquarium water and the low pH. The pH of the synthetic freshwater (Table 2) was lower than the “standard, synthetic freshwater “ prepared with sodium bicarbonate, calcium sulfate and magnesium sulfate according to the USEPA document (EPA/600/4-90/027, September 1991). The expected equilibrium pH after 24 hr aeration for the standard very soft water is 6.4 to 6.8. Relatively few laboratory studies have been conducted with fathead minnows maintained in aquaria with pH below 6.5. In a feeding study with fathead minnow fry, Duda and Butner (28) maintained the fry for 30 days (hatch to post-hatch) at pH 6.0 - 7.4 in flow through containers and the water quality was considered generally acceptable for fish survival and growth. Using very soft diluted Lake Superior water acidified with sulfuric acid (hardness approximately 4.5 mg/L, Leino et al. (29) obtained morphological evidence for chloride cell damage in adult fathead minnows after 9 days of exposure to pH 5.0.
Our survival results are consistent with the findings of Grosell et al (19) with larval fathead minnows. These workers found that lead toxicity is substantially increased in larval fish at pH 6.7 relative to a control pH of 7.4. Van der Putte and coworkers (30) found that reducing the pH from 7.8 to 6.5 also increased the toxicity of chromium VI several fold in rainbow trout Salmo gairdneri. Their findings suggested that the gills were the chief target organ of chromium toxicity at a pH of 6.5. Gill tissue also accumulates higher chromium levels relative to other organs and tissues, according to several reports (30, 31).
Our results raise the possibility of a synergistic toxic effect of divalent lead cations and low pH. Based on studies of cadmium toxicity in freshwater fish, other investigators (32) have suggested that low pH at the gill microenvironment might promote uptake of the divalent cation, thereby increasing bioavailablity. Like cadmium salts, precipitation of lead salts as a metal complex on the external gill surface is more likely in a hard water environment. Taken together with this work by others, our results suggest that lead uptake, possibly through high affinity calcium channels in gill epithelium, is enhanced at low pH. A general mechanism for interaction of metal cations with certain epithelial components of teleost gills has been described by Reid and Macdonald (33), although the nature of the epithelial molecules; e.g., transporter proteins or epithelial cell-cell junctions (34), remains obscure.
The reason for the comparatively high pH in aquarium C is unknown. We anticipated that the biological filtration systems would not operate well with the very soft water, but, conceivably, the filter of aquarium C may have been colonized by bacteria which were less effective in oxidizing NH4+ to NO3-. This nitrification process is thought to result in gradual acidification, and may have contributed to the acidity in all aquaria.
The low pH in aquarium D may have augmented lead toxicity, but the deaths cannot be attributed to acidity alone. We elected to use a very soft SFW formulation, with hardness <15 mg/L, as calcium ions are known to compete with Pb++ for epithelial uptake (35,36). Many lead toxicity studies have been carried out with fathead minnows at different hardness levels. According to work cited in the US Fish and Wildlife Contaminant Hazard Review monograph by Eisler (12), when fathead minnows were exposed to lead (96 h exposure duration) at hardness levels of 20 and 360 mg CaCO3/L, the LC50 values for the medium concentration of lead were 6.5 and 460 ppm, respectively. Evidence from other acute (24 - 96 h) studies with fathead minnows indicates that low water hardness (≤20 mg CaCO3/L) potentiates Pb++ toxicity (Legislative Report, Minnesota Pollution Control Agency, 1999). This effect of low hardness has been seen regardless of aquarium design (static or flow through) or the lead salt tested (chloride or acetate).
Previous toxicity studies (12, and those cited in Legislative Report, Minnesota Pollution Control Agency, 1999), with fathead minnows have typically employed lead concentrations well above 1 ppm. Our in vitro results indicate that such high concentrations would be expected to result in lipid peroxidation of gill membranes. To our knowledge, this is the first study showing lead-induced oxidative stress to fathead minnow gills as evidenced by reduction of the GSH/GSSG ratio. However, Dandapat et al. (37) monitored formation of thiobarbituric acid reactive substances by invertebrate gills, and found that certain metal ions, notably divalent cadmium and ferric ions, are potent in causing lipid peroxidation of gills in vitro. We speculate that, in the aquarium D fish of our study, lead may have accumulated in the gills to the extent that a large oxidant stress was generated, resulting in compromised gas transport and disturbances of ionoregulation. In summary, our aquarium studies show that rather low concentrations of lead, near 200 ppb, can be lethal to fathead minnows in a low Ca++, low Mg++ environment. Further work is needed to understand the interaction between Pb++ and Ca++ in gill epithelium, and to determine if lipid peroxidation of gill membranes contributes to the enhancement of lead toxicity seen in fathead minnows at low water hardness.
Acknowledgments
This publication was made possible by AREA grant number 2R15ES009434-02A1 from the National Institute of Environmental Health Sciences (NIEHS), NIH. It’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. We are indebted to Bertram Lipitz, DVM, and his staff at the UMDNJ-SOM Research Animal Facility for many helpful suggestions. We thank Dr. Michael S. Wolin (New York Medical College) for useful discussions relating to evaluation of glutathione status. We are grateful for the technical advice of Susan Grau who assisted with the fish husbandry. We appreciate the assistance of Jaime M. Levine, M.D., who helped with the in vitro studies. We thank Barbara S. Spokas, B.S., Robert Wood Johnson School of Allied Health, for computer graphics assistance.
Literature Cited
- (1).Ankley GT, Jensen KM, Durhan EJ, Makynen EA, Butterworth BC, Kahl MD, Villeneuve DL, Linnum A, Gray LE, Cardon M, Wilson VS. Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas) Toxicol. Sci. 2005;86:300–308. doi: 10.1093/toxsci/kfi202. [DOI] [PubMed] [Google Scholar]
- (2).Weber DN. Exposure to sublethal levels of waterborne lead alters reproductive behavior in fathead minnows (Pimephales promelas) Neurotoxicology. 1993;14(23):347–358. [PubMed] [Google Scholar]
- (3).Weber DN, Russo A, Seale DB, Spieler RE. Waterborne lead effects feeding abilities and neurotransmitter levels of juvenile minnows (Pimephales promelas) Aquatic Toxicol. 1991;21(12):71–80. [Google Scholar]
- (4).Harris HH, Pickering IJ, George GN. The Chemical Form of Mercury in Fish. Science. 2003 August;301(Issue 5637):1203–1203. doi: 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- (5).Mahaffey KR, Clickner RP, Bodurow CC. Blood Organic Mercury and Dietary Mercury Intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hollis L, Hogstrand C, Wood CM. Tissue-specific cadmium accumulation, metallothionein induction, and tissue zinc and copper levels during chronic sublethal cadmium exposure in juvenile rainbow trout. Arch. Environ. Contam. Toxicol. 2001;41:468–474. doi: 10.1007/s002440010273. [DOI] [PubMed] [Google Scholar]
- (7).Ay O, Kalay M, Tamer L, Canli M. Copper and lead accumulation in tissues of a freshwater fish Tilapia zillii and its effects on the branchial Na, K-ATPase activity. Bull. Environ. Contam. Toxicol. 1999;62:160–168. doi: 10.1007/s001289900855. [DOI] [PubMed] [Google Scholar]
- (8).Lohner TW, Reash RJ, Willet V.Ellen, Rose LA. Assessment of tolerant sunfish populations (Lepomis sp.). 1. Hematological and population level assessment. Ecotoxicol. Environ. Saf. 2001;50:203–216. doi: 10.1006/eesa.2001.2097. [DOI] [PubMed] [Google Scholar]
- (9).Rashed MN. Monitoring of environmental heavy metals in fish from Nasser Lake. Environ. Int. 2001;27:27–33. doi: 10.1016/s0160-4120(01)00050-2. [DOI] [PubMed] [Google Scholar]
- (10).Venuto CJ, Trefry JH. Frequency distribution patterns and partitioning of copper, iron and zinc in selected tissues of the black mullet (Mugil cephalus) Florida Sci. 1983;46:346–356. [Google Scholar]
- (11).Sola F, Masoni A, Isaia J. Effects of lead loads on branchial osmoregulatory mechanisms in the rainbow trout Oncorhynchus mykiss. J. Applied Toxicol. 1994;14(5):343–349. doi: 10.1002/jat.2550140505. [DOI] [PubMed] [Google Scholar]
- (12).Eisler R, U.S. Fish and Wildlife Service Lead hazards to fish, wildlife and invertebrates: A synoptic review. Contaminant Hazard Rev. 1988 April;(14) Biological report 85(1.14) [Google Scholar]
- (13).Hochheimer JN. “Water Chemistry in Recycle Systems”. Workshop on “Management of Aquatic Animal Facilities”; Seminar presented at the American Academy of Laboratory Animal Science Annual meeting; Anaheim CA. November 18, 1997. [Google Scholar]
- (14).Penning W, Scoppa P, Directorate General XII of the Commission of the European Communities . Biological reactive intermediates. 1977. pp. 448–451. Contribution No.1230 of the Biology Programme. Degradation of Cytochrome P450 Heme and Lipid Peroxidation in Lead-Poisoned Rats. [Google Scholar]
- (15).Sandhir R, Julka D, Gill KD. Lipoperoxidative damage on lead exposure in rat brain and its implications on membrane-bound enzymes. Pharmacol and Toxicol. 1994;74:66–71. doi: 10.1111/j.1600-0773.1994.tb01077.x. [DOI] [PubMed] [Google Scholar]
- (16).Sandhir R, Gill KD. Effect of lead on lipid peroxidation in liver of rats. Biol. Trace Elem. Res. 1995;48:91–97. doi: 10.1007/BF02789081. [DOI] [PubMed] [Google Scholar]
- (17).Spokas EG, Crivellone MD, Kemp F, Bogden JD, Cohen GM. Characterization of Sodium, Potassium, ATPase Activity in the Gills of Pimephales promelas (Fathead Minnow): Influence of In Vitro Exposure to Lead. Bull. Environ. Contam. Toxicol. 2002;69:384–392. doi: 10.1007/s00128-002-0074-x. [DOI] [PubMed] [Google Scholar]
- (18).Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- (19).Grosell M, Gerdes R, Brix KV. Influence of Ca, humic acid and pH on lead accumulation and toxicity in the fathead minnow during prolonged water-borne lead exposure. Comp. Biochem. Physiol. C, Toxicol. Pharmacol. 2006 doi: 10.1016/j.cbpc.2006.04.014. Epub ahead of print May 6, 2006. [DOI] [PubMed] [Google Scholar]
- (20).Malins DC, Stegeman JJ, Anderson JW, Johnson PM, Gold J, Anderson KM. Structural Changes in Gill DNA Reveal the Effects of Contaminants on Puget Sound Fish. Environ Health Perspect. 2004 April;112:511–515. doi: 10.1289/ehp.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).DiToro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC. Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ. Toxicol. Chem. 2001;20:2383–2396. [PubMed] [Google Scholar]
- (22).Macdonald A, Silk L, Schwartz M, Playle RC. A lead-gill binding model to predict acute lead toxicity to rainbow trout (Oncorhynchus mykiss) Comp. Biochem. Physiol. Part C. 2002;133:227–242. doi: 10.1016/s1532-0456(02)00107-2. [DOI] [PubMed] [Google Scholar]
- (23).Preslan JE, Chang C-Y, George WJ. Bioavailability of lead to fish from waters containing a vitrified slagged aggregate (VSA) The Toxicologist. 1993;233 Abstract. [Google Scholar]
- (24).Thomas P, Juedes MJ. Influence of lead on the glutathione status of Atlantic croaker tissues. Aquat. Toxicol. 1992;23:11–30. [Google Scholar]
- (25).Eisler R, U.S. Fish and Wildlife Service Mercury hazards to fish, wildlife and invertebrates: A synoptic review. Contaminant Hazard Rev. 1987 April;(10) Biological report 85(1.10) [Google Scholar]
- (26).Raghavan SRV, Culver BD, Gonick HC. Erythrocyte lead-binding protein after occupational exposure. II. Influence of lead inhibition of membrane Na+, K+ - adenosine triphosphatase. J. Toxicol. Environ. Health. 1981;7:561–568. doi: 10.1080/15287398109530001. [DOI] [PubMed] [Google Scholar]
- (27).Goyer RA, Clarkson TW. Toxic Effects of Metals. In: Klaasen CD, editor. Toxicology, The Basic Science of Poisons, Cassarett and Douhl’s Toxicology. 6th Edition. McGraw-Hill; Medical Publishing Division, NY: 2001. pp. 811–867. Chapter 23. [Google Scholar]
- (28).Duda SW, Buttner JK. Reproduction, survival, and growth of fathead minnow fry in the laboratory. Lab Animal. 1993 February;:40–46. [Google Scholar]
- (29).Leino RL, McCormick JH, Jensen KM. Changes in gill histology of fathead minnows and yellow perch transferred to soft water or acidified soft water with particular reference to chloride cells. Cell and Tissue Research. 1987;250(2):389–399. [Google Scholar]
- (30).Van der Putte I, Brinkhorst MA, Koeman JH. Effect of pH on the acute toxicity of hexavalent chromium to rainbow trout (Salmo gairdneri) Aquat. Toxicol. 1981;1:129–142. [Google Scholar]
- (31).Van der Putte I, Lubbers J, Kolar Z. Effect of pH on uptake, tissue distribution and retention of hexavalent chromium in rainbow trout (Salmo gairdneri) Aquat. Toxicol. 1981;1:129–142. [Google Scholar]
- (32).van Aardt WJ, Booysen J. Water hardness and the effects of Cd on oxygen consumption, plasma chlorides and bioaccumulation in Tilapia sparrmanii. Water SA. 2004;30(1):57–64. [Google Scholar]
- (33).Reid SD, MacDonald DG. Metal binding activity of the gills of the rainbow trout (Onchorhynchus mykiss) Can. J. Fish. Aquat. Sci. 1991;48:1061–1068. [Google Scholar]
- (34).Niewenhuis RN, Dimitriu C, Prozialeck WC. Ultrastructural characterization of the early changes in intercellular junctions in response to cadmium (Cd+2) exposure in LLC-PK1 Cells. Toxicol. Appl. Pharmacol. 1997;142:1–12. doi: 10.1006/taap.1996.8026. [DOI] [PubMed] [Google Scholar]
- (35).Goyer RA. Toxic and essential metal interactions. Ann. Rev. Nutr. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- (36).Han S, Pfizemaier DH, Garcia E, Eguez ML, Ling M, Kemp FW, Bogden JD. Effects of lead exposure before pregnancy and dietary calcium during pregnancy on fetal developments and lead accumulation. Environ. Health Perspect. 2000;108:527–531. doi: 10.1289/ehp.00108527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Dandapat J, Janardhana Rao K, Chainy GBN. An in vitro study of metal ion- induced lipid peroxidation in giant fresh water prawn Macrobrachium rosenbergii (de MAN) Biometals. 1999;12:89–97. [Google Scholar]


