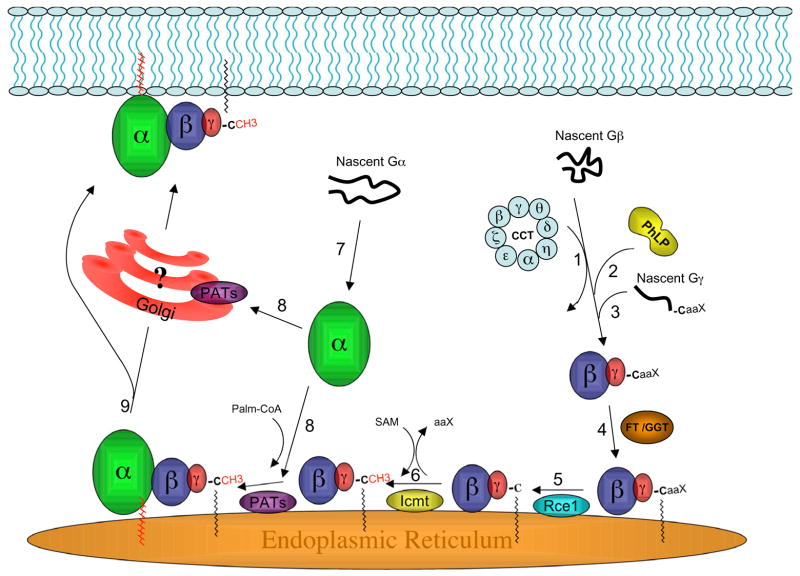
Figure 1. Model of G protein assembly and trafficking to the plasma membrane.
Recent studies have suggested a model for the plasma membrane targeting of nascent G protein subunits. The following key steps are indicated in the figure and further discussed in the text: 1) The CCT chaperone complex binds to nascent Gβ and promotes proper folding. 2) PhLP1 binds to form a ternary complex, and then PhLP1 phosphorylation stimulates the release of PhLP1-Gβ. 3) Nascent Gγ then binds to form PhLP1-Gβγ. It is not clear when and where PhLP1 and Gβγ dissociate. In addition, proteins such as DRiP78 may facilitate folding of Gγ. 4) Gγ is farnesylated or geranylgeranylated by a cytoplasmic farnesyl transferase or geranylgeranyl transferase (FT/GGT), and then Gβγ is targeted to the cytoplasmic surface of the ER. 5) An ER-localized protease (Rce or ras converting enzyme) specifically cleaves the C-terminal three amino acids (i.e, the –aaX of the CaaX motif) from isoprenylated Gγ. 6) The new isoprenylcysteine carboxyl terminus of Gγ is methylated by a specific ER-localized carboxyl methyl transferase (Icmt). 7) Similar to Gβ, chaperone proteins may exist to promote proper folding and membrane targeting of Gα. Ric-8, or similar proteins, has been speculated to play such a role for Gα; however, this remains to be defined. 8) Unanswered questions include: exactly where in the cell Gα and Gβγ interact to form the heterotrimer and where Gα is palmitoylated. Specific palmitoyl acyl transferases (PATs) have not been clearly defined for Gα, although many PATs are localized at Golgi or ER membranes. Based on several studies (see text), we speculate that the ER is a site of heterotrimer formation and Gα palmitoylation. 9) Lastly, in this model the newly formed heterotrimer moves from an intracellular location to the PM. The details of this process are mostly unknown. Some evidence supports a Golgi-independent pathway, but a role for the Golgi cannot be ruled out.