Figure 7. Effect of reducing the lipid-protein molar ratio for DOPA/CH membranes on the photoincorporation of [125I]TID into subunits of the nAChR.
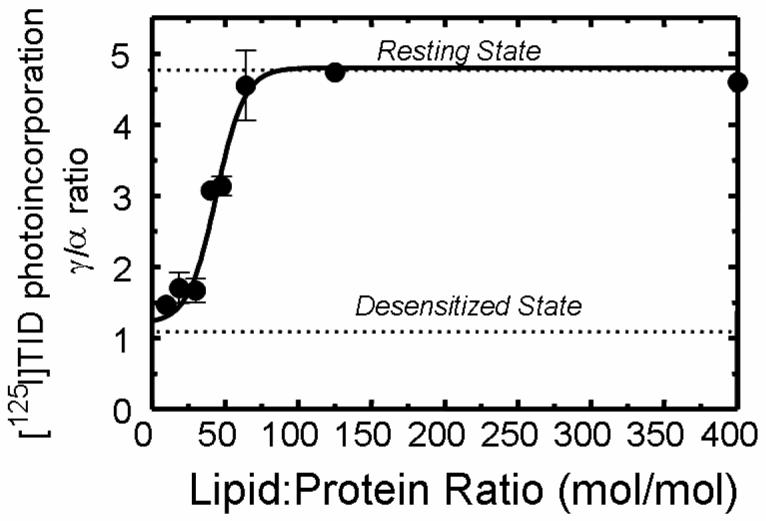
Affinity-purified nAChRs were reconstituted into membranes comprised of DOPA/CH (1:1) in which the overall lipid-protein molar ratio was adjusted to 400:1, 125:1, 65:1, 48:1, 45:1, 30:1, 18:1, and 9:1 respectively. The functionality of the nAChR was determined using [125I]TID labeling as described in Experimental Procedures. Briefly, samples were equilibrated for 1 h with [125I]TID (0.4 μM) in the absence (− lanes) and in the presence (+ lanes) of 400 μM Carb, irradiated at 365 nm for 7 min, and the polypeptides resolved by SDS-PAGE. Labeled nAChR subunit bands were excised from the dried gel and the amount of [125I]TID photoincorporated into each subunit determined by γ counting (5 min of counting time). [125I]TID incorporation into γ' was added to that of the γ-subunit. The ratio of [125I]TID labeling in the γ– and α–subunit in the absence of agonist was calculated. Shown is the relationship between the lipid-protein molar ratio and the functionality of nAChR as indicated by the γ/α labeling ratio (error bars indicate the standard error). The γ/α ratio points (●) are means of three different [125I]TID labeling experiments (error bars indicate standard error, see also supporting information). For comparison, the γ/α ratios for nAChRs fully stabilized in the resting and desensitized states are indicated with a dotted line
