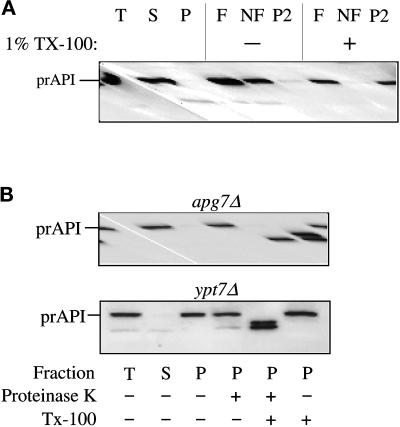
Figure 3.
The apg7Δ mutant accumulates precursor API in a membrane-associated and protease-sensitive form that is part of a large complex. (A) The apg7Δ strain (VDY1) was grown in SMD to midlog phase and converted to spheroplasts. The spheroplasts were lysed in osmotic lysis buffer (see MATERIALS AND METHODS). An aliquot was removed for a total lysate control (T). The remainder of the lysed spheroplasts were separated into supernatant (S) and pellet (P) fractions by centrifugation at 5000 × g. The pellet fraction was resuspended in 60% sucrose in GB in the presence or absence of Triton X-100 and overlaid with 55 and 35% sucrose in GB. The step gradients were centrifuged at 100,000 × g for 60 min. Membrane-containing float (F), nonfloat (NF), and pellet (P2) fractions were collected and subjected to immunoblot analysis with antiserum to API as described in MATERIALS AND METHODS. The position of precursor API is indicated. (B) Precursor API in apg7Δ is protease accessible. Spheroplasts isolated from apg7Δ (VDY1) and ypt7Δ (WSY99) cells were lysed in osmotic lysis buffer. Supernatant (S) and pellet (P) fractions after a 5000 × g centrifugation were collected, and the pellet fractions were subjected to protease treatment in the absence or presence of 0.2% Triton X-100 as described in MATERIALS AND METHODS. The resulting samples were subjected to immunoblot analysis with antibody against API.