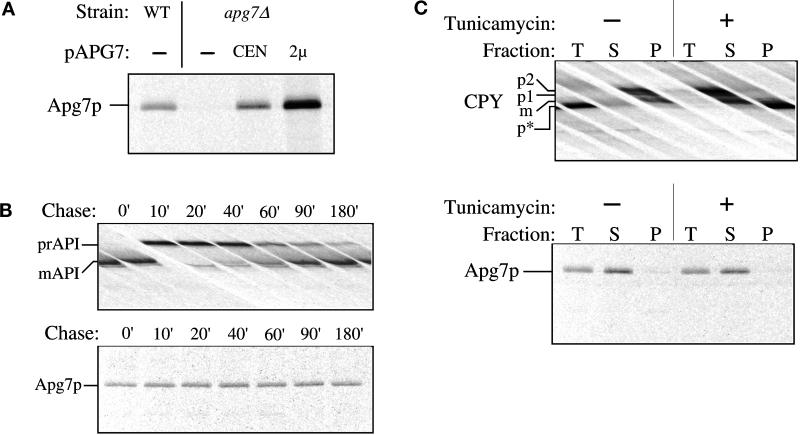
Figure 4.
Apg7p is not glycosylated or proteolytically modified. (A) WT (wild-type, SEY6210), apg7Δ (VDY1) and the apg7Δ strain transformed with single-copy (CEN) or multicopy (2 μ) plasmids encoding APG7 were grown to log phase in SMD. Cells were labeled for 10 min and immunoprecipitated with antiserum to Apg7p and analyzed by SDS-PAGE as described in MATERIALS AND METHODS. Apg7p is detected as a 71-kDa protein. (B) Wild-type (SEY6210) cells were radiolabeled for 10 min and subjected to a nonradioactive chase. At the indicated time points an aliquot was removed and precipitated with TCA. Protein extracts were prepared and successively immunoprecipitated with antiserum to API and Apg7p and analyzed as above. The positions of precursor and mature API and of Apg7p are indicated. The absence of a molecular mass shift indicates that Apg7p is not proteolytically modified. (C) Wild-type (SEY6210) cells were converted to spheroplasts and treated with tunicamycin (final concentration 20 μg/ml) to inhibit glycosylation 15 min before the addition of radioactive label as indicated. Labeling was allowed to continue for 20 min. Samples were TCA precipitated and divided in half. One-half was immunoprecipitated immediately with antiserum to CPY or Apg7p for a total (T) control. The remaining half was precipitated with Con A-Sepharose and separated into supernatant (S, not bound to Con A) and pellet (P, bound to Con A) fractions as referenced in MATERIALS AND METHODS. The separate fractions were then immunoprecipitated with antiserum to CPY or Apg7p. The positions of precursor and mature forms of glycosylated CPY (p1, p2, m) and unglycosylated precursor CPY (p*) and of Apg7p are shown. Apg7p does not bind Con A, suggesting that it is not glycosylated.