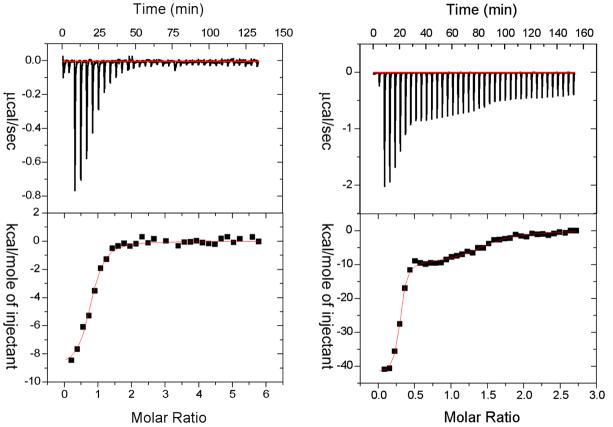
Figure 3.
Isothermal titration calorimetric studies for the binding of benzenesulfonamide (left panel) and BR30 (right panel) to CAII. The top panels show the raw data for the titration of 10 μM enzyme with increasing aliquots (4 μl each) of stock solution of the ligands. The bottom panels show the integrated heat signals as a function of molar ratios of ligands to CAII. The solid smooth lines are the best fit of the data for “one site” (left panel) and “two site” (right panel) classes of binding sites of benzenesulfonamide and BR30 to CAII, respectively. Following are the derived thermodynamic parameters for the best fit of the data. CAII-benzenesulfonamide complex (one binding site model): N = 0.76, Ka = 1.7 × 106 M−1, and ΔH° = −9.1 kcal/mol. CAII-BR30 complex (two binding site model): N1 = 1.2, Ka1 = 2.9 × 108 M−1, ΔH°1 = −42.2 kcal/mol; N2 = 0.28, Ka2 = 1.6 × 106 M−1, ΔH°2 = −9.8 kcal/mol.