Scheme I.
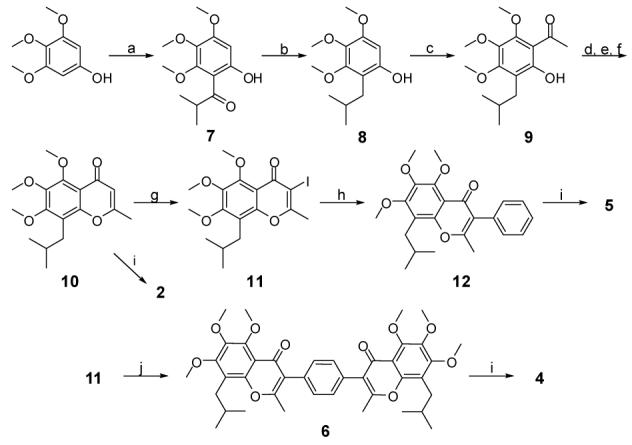
Synthesis of designed compounds 2, 4, 5 and 6a. Reagents and conditions: (a) isobutyric chloride, BF3·Et2O, Cl(CH2)2Cl, reflux, 85%; (b) Et3SiH, TFA, 95%; (c) AcCl, BF3·Et2O, Cl(CH2)2Cl, reflux, 87%; (d) Ac2O, pyridine; (e) NaH, DMF; (f) HCl, 82% f or 3 steps; (g) I2, CF3CO2Ag, CH2Cl2, 0 °C, 94%; (h) PhB(OH)2, Pd2(dpf )2Cl2·CH2Cl2, Na2CO3, EtOH, DMF, H2O, 60 °C, 92%; (i) BBr3, CH2Cl2, -78 °C ∼ 0 °C; (j) 1,4-phenylenebisboronic acid (0.5 eq.), Pd2(dpf)2Cl2·CH2Cl2,Na2CO3, EtOH, DMF, H2O, 90 °C, 96%.
