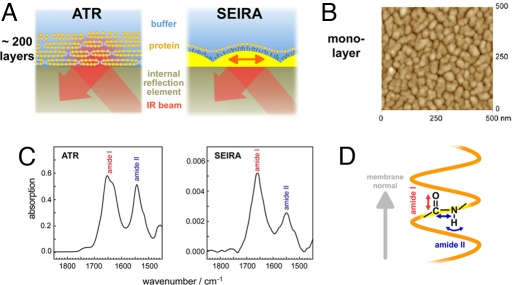
Fig. 1.
Infrared spectroscopy on membrane proteins. (A) ATR spectroscopy probes stacks of several hundred membrane layers by the evanescent IR field atop an internal reflection element. The large number of membrane layers enables sufficiently high signal but hampers ligand binding or application of membrane potentials. In SEIRA spectroscopy, the IR field is enhanced by a nanostructured Ni-NTA modified gold film because of excitation of surface plasmons, allowing spectroscopic studies on a self-assembled membrane protein monolayer, selectively. (B) Atomic-force microscopic image of the rough gold surface used for SEIRA. The diameter of the gold nanoparticles is ≈50 nm with a height of 10 nm atop the continuous gold layer of 20 (±10) nm thickness. (C) ATR/FT-IR absorption spectra of a stack of the membrane protein sensory rhodopsin II reconstituted in halobacterial lipids (Left) in comparison with the SEIRA spectrum of a monolayer of SR II tethered to the modified gold surface via a His-tag (Right). The spectra have been corrected for vibrational contributions from the solvent water (scissoring mode of H2O at ≈1,645 cm−1). (D) Differing intensities of amide I and II vibrational modes of the protein backbone are due to their microscopic orientation and the polarization of the enhanced field with respect to the membrane normal.