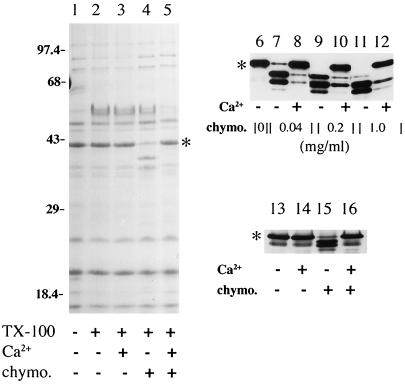
Figure 7.
Chymotryptic digestion of Grl1p in isolated granules and cell homogenates. In all three panels, ∗ indicates the position of mature Grl1p. Several of the smaller Grlps cannot be seen on this gel; their chymotrypsin insensitivity was noted in other experiments using higher percentage gels. Lanes 1–5: Aliquots of isolated granules were treated as described in the table beneath the lanes; ∼40 μg of granule protein was loaded per lane of a 15% polyacrylamide gel. In the presence of Triton X-100, chymotrypsin (1 mg/ml) generated distinct fragments of Grl1p (lane 4). Incubation with calcium (1 mM) before addition of chymotrypsin resulted in protection of Grl1p (lane 5). Samples were visualized with Coomassie blue. Lanes 6–12. Isolated granules were incubated with 1% Triton X-100, and aliquots containing 8 μg of protein, were treated as indicated in the table beneath the lanes. After SDS-PAGE, proteins were transferred to nitrocellulose and antibody blotted with antiGrl1p. A ladder of proteolytic products was generated with increasing concentrations of chymotrypsin (lanes 7, 9, 11). At all chymotrypsin concentrations, preincubation with 1 mM calcium provided extensive protection (lanes 8, 10, 12). Lanes 13–16. Aliquots of a crude particulate fraction from MN173 cells were treated as indicated. One hundred and fifty micrograms of total protein was loaded per lane; the chymotrypsin concentration was 0.5 mg/ml. Proteins were visualized by antibody blotting with anti-Grl1p.