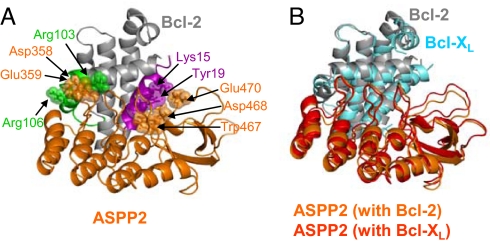
Fig. 3.
Docking model for the interaction between the ASPP2Ank-SH3 and Bcl-2/Bcl-XL proteins. (A) Bcl-2 binding to ASPP2Ank-SH3. Bcl-2 is colored gray, the BH4 site is in magenta, and the proapoptotic site is in green. ASPP2Ank-SH3 is colored orange. (B) Two aligned models of Bcl-2 and Bcl-XL complexes with ASPP2Ank-SH3. Bcl-XL is colored cyan, and Bcl-2 is in gray. ASPP2Ank-SH3 is colored red and orange for its complex models with Bcl-XL and Bcl-2, respectively. Representative binding residues from both partners are depicted in sticks and spheres. The conserved interactions between Bcl-2 (PDB entry 1YSW) and ASPP2 (PDB entry 1ycs) are as follows: K15–D468 and E470; H18–D468 and E481; Y19–W467; K20–D468 and E481; R24–D352; R103–D358 and D359; Y105–P356; and R106–D333, D358, and E359. The homologous amino acids in Bcl-XL show the same interactions with ASPP2. For consistency with the PDB file, numbering of the ASPP2 residues is according to the sequence of its truncated form 53BP2.