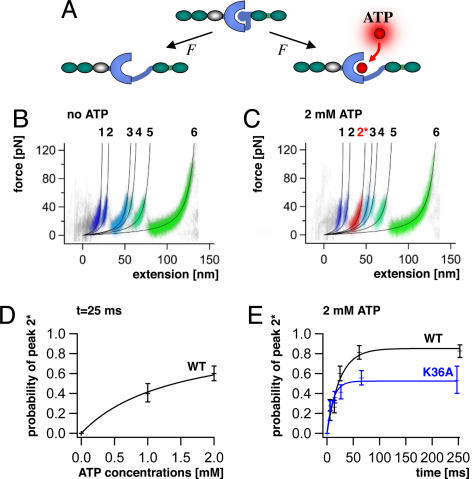
Fig. 2.
Unfolding profile of TK and kinetics of mechanically induced ATP binding. (A) External force can open the ATP binding site of TK by unfolding of the autoinhibitory domain (blue ball). (B) Superimposed traces of 66 single-molecule unfolding events in TK show a fixed sequence of local unfolding events, numbered 1–5. (C) Mechanically induced ATP binding leads to a distinctly altered force profile with the appearance of an extra force peak, 2*, absent in unfolding events in the absence of ATP (44 traces). (D) ATP binding probability (i.e., the occurrence of peak 2*) depends on ATP concentrations, giving access to virtual direct kinetics though the system is not in equilibrium (ATP peaks detected at pulling rates of 720 nm/s, or after 25 ms). (E) Probability of ATP binding depends on pulling rates, decreasing at faster speeds because of reduced opening time of the ATP binding site. ATP binding is strongly reduced by mutation of lysine-36 to alanine (K36A).