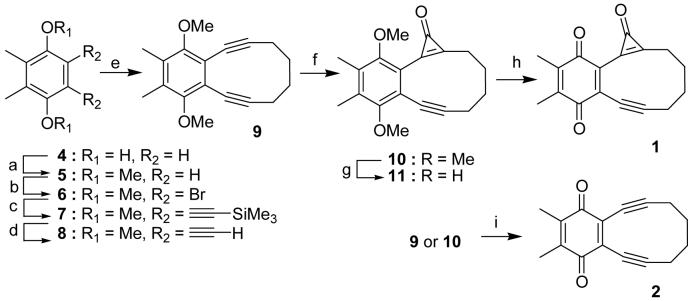
Scheme 2.
Reagents and conditions: (a) Me2SO4, K2CO3, acetone; (b) Br2, CHCl3, 77% (two steps); (c) HC≡CSiMe3, Pd(PPh3)2Cl2, Cul, PPh3, piperidine; (d) K2CO3, MeOH, 71% (two steps); (e) n-BuLi, I(CH2)4I, THF, HMPA, - 78°→r.t., 42%; (f) CHCl3, n-BuLi, THF, -78°C, 86%; (g) BBr3, CH2Cl2, - 78°C→r.t.; (h) FeCl3, THF, 23% (two steps); (i) CAN in aq. acetonitrile, 81% (10) or 89% (9)