FIGURE 6. Two dimensional gel electrophoresis analysis of plasma of apoA-I-/- mice expressing the WT or mutant forms of apoA-I or the control protein GFP. Agarose gel electrophoresis analysis of plasma of apoA-I-/- mice expressing the WT apoA-I or the apoA-I[Δ(185-243)] mutant and of purified apoA-I[Δ(185-243)] protein.
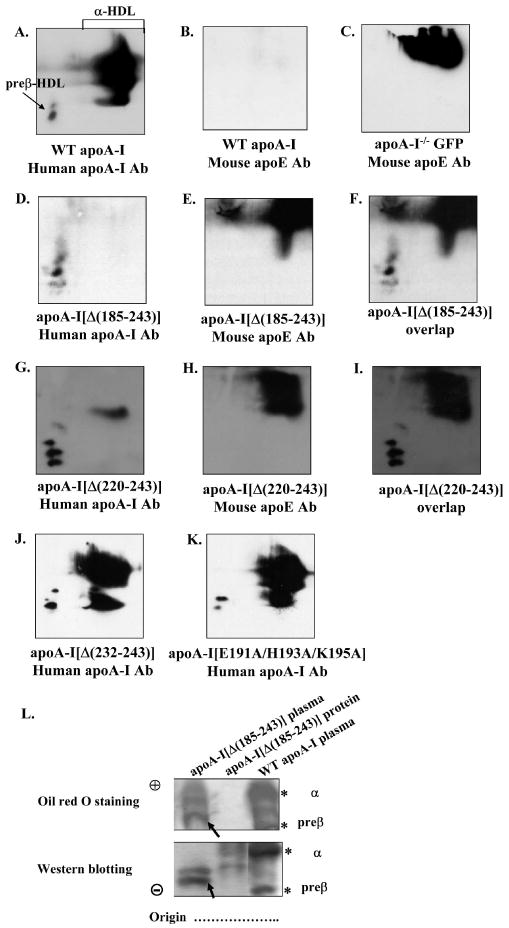
The plasma of mice expressing the WT apoA-I (A, B) or the control protein GFP (C) or the carboxy-terminal mutants apoA-I[Δ(185-243)] (D-F), apoA-I[Δ(220-243)] (G-I), apoA-I[Δ(232-243)] (J), apoA-I[E191A/H193A/K195A] (K) were analyzed by two dimensional gel electrophoresis and Western blotting using anti-human apoA-I antibody (A, D, G, J, K) or anti-mouse apoE antibody (B, E, H), as described in Experimental Procedures. Panels F and I show the overlapping of panels D, E and G, H, respectively. Panel L, The plasma of mice expressing the WT apoA-I or the carboxy-terminal mutant apoA-I[Δ(185-243)] and purified apoA-I[Δ(185-243)] protein were analyzed by 0.7% agarose gel electrophoresis followed by Oil Red O neutral lipid staining or Western blotting using a goat polyclonal anti-human apoA-I antibody as described in Experimental Procedures. The asterisks indicate preβ- and α-HDL that contain WT apoA-I. The apoA-I[Δ(185-243)] mutant formed particles with faster electrophoretic mobility than the preβ-HDL of WT apoA-I as demonstrated by Western blot analysis, that accumulated significant levels of neutral lipid (bands indicated by arrow).
