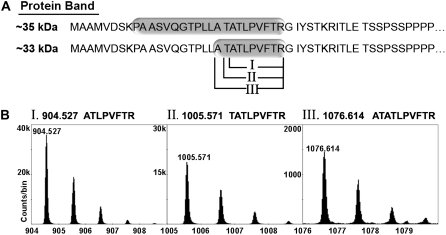
Figure 5.
MS-based identification of precursor and mature Chlase versions and the N-terminal processing sites. Mature green lemon fruit were treated with ethylene (20 μL L−1) at 25°C in the dark for 12 or 120 h in a 600-L sealed container. The container was ventilated once every 24 h, followed by injection of fresh ethylene, to maintain CO2 levels below 1%. The flavedo of the treated and control fruit was peeled, frozen in liquid nitrogen, and ground to a powder. Total protein from each time point was acetone precipitated, extracted in RIPA buffer, and Chlase versions were immunoprecipitated using affinity-purified anti-citrus Chlase antibodies. Putative precursor (approximately 35 kD) and mature (approximately 33 kD) Chlase versions were separated from the antibody by SDS-PAGE, visualized by colloidal Coomassie staining, and excised from the gel. Protein bands were digested with trypsin in-gel and eluted peptides were analyzed by MS/MS. A, The N-terminal tryptic and semi-tryptic peptides detected after trypsinization of the approximately 35-kD and 33-kD protein bands, respectively. Tryptic and semi-tryptic peptides are highlighted on the N-terminal amino acid sequence of Chlase (starting at Met-1). Roman numerals (I, II, III) designate the three different semi-tryptic peptides detected. B, Semi-tryptic peptides I, II, and III observed in the TOF mass spectrum of the HPLC fraction that eluted at 23 min. In each case, the multiple peaks correspond to the normal isotopic distribution (i.e. to the inclusion of 0, 1, 2, or 3 13C atoms in the peptide molecule). Mass of the monoisotopic peaks (zero 13Cs) appears above each peak and above each together with the sequences that were confirmed by MS/MS measurements (Table I). Note the different vertical scales in the three segments; all three peptides were observed in the same mass spectrum, collected at the same time under identical instrumental conditions, so the range of observed intensities of the monoisotopic peaks in the three peptide spectra (from approximately 1,500 counts/bin to almost 40,000 counts/bin) provides an estimation of the quantity of each of the detected peptides.