Fig. 5.
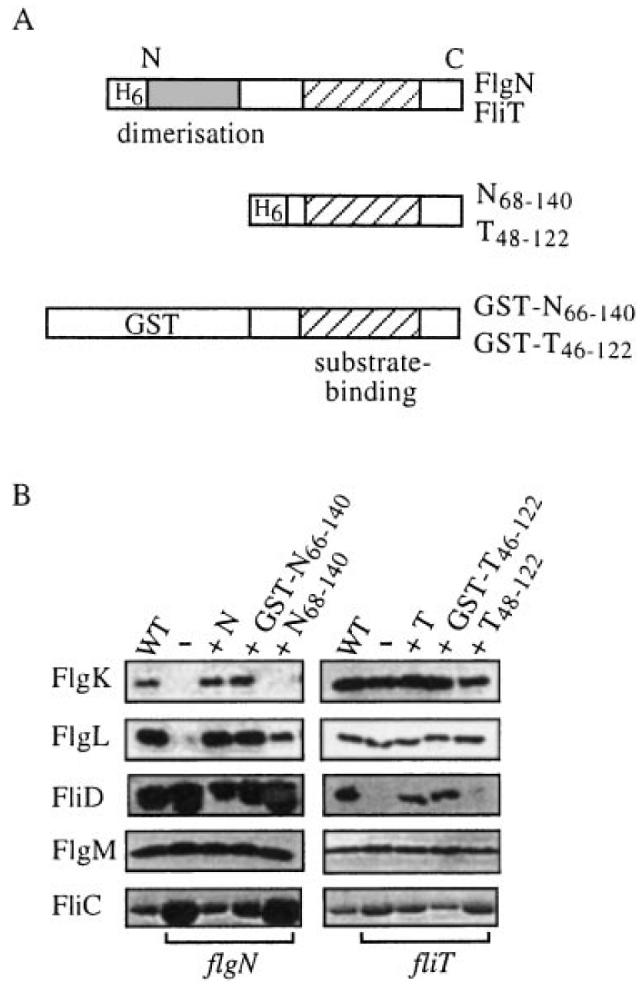
Complementation of flgN and fliT mutants by chaperone C-terminal regions.
A. Representations of (top) FliT and FlgN indicating putative N-terminal dimerization (shaded) and C-terminal substrate-binding (hatched) domains; (middle) the FlgN and FliT N-terminally histidine-tagged (H6) polypeptides; and (bottom) GST translational fusions. The C-terminal amphipathic helix is predicted to span amino acids 74–114 in FlgN and residues 60–98 in FliT. The predicted FlgN leucine zipper is located between residues 1 and 30.
B. Flagellar proteins collected from the supernatants of late exponential phase flgN (left) or fliT (right) mutant cultures alone (–) or expressing either His-tagged FlgN or FliT (labelled N and T) or the respective GST fusion or the C-terminal polypeptide. The same proteins exported by WT S. typhimurium are shown in each case in the leftmost columns. Proteins separated by SDS–(12%)PAGE were immunoblotted with anti-FlgK, anti-FlgL, anti-FliD or anti-FlgM antisera or by Coomassie blue staining (FliC).
