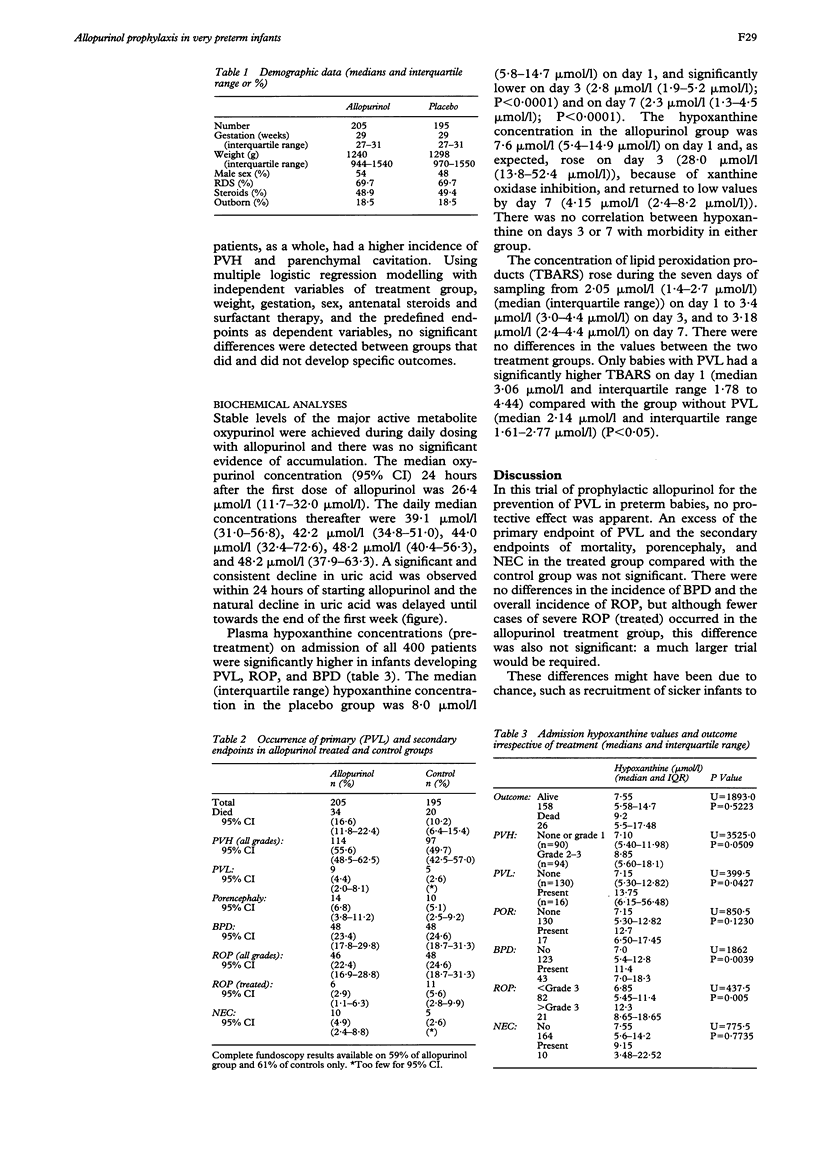
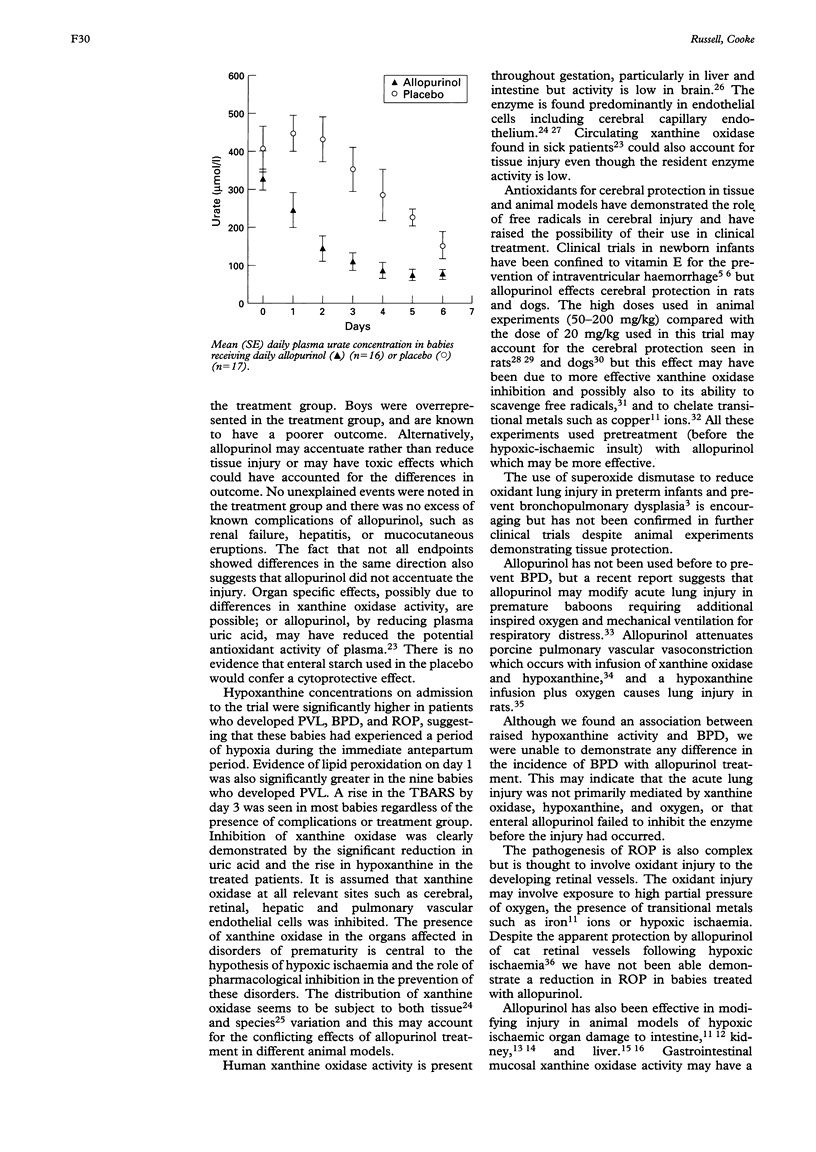
Abstract
Allopurinol, an inhibitor of xanthine oxidase (an enzyme capable of generating superoxide radicals following hypoxiaischaemia), was investigated in preterm infants to determine its ability to prevent the complications of neonatal intensive care which may be associated with oxidative injury. Four hundred infants of between 24 and 32 weeks' gestation were randomly allocated to receive enteral allopurinol (20 mg/ml) or an equivalent dose of placebo for seven daily doses. At admission, plasma hypoxanthine concentrations were significantly higher in infants who subsequently developed periventricular leucomalacia (PVL), bronchopulmonary dysplasia (BPD), or retinopathy of prematurity (ROP), but there was no difference in the primary endpoint (PVL) between the treated and control groups. The failure of allopurinol prophylaxis in this group of infants is probably related to the complex nature of the pathological processes and to the timing of treatment. If oxidant injury is an important mechanism of cellular injury in these preterm infants, an alternative biochemical modulator would be required, or a combination of agents might be effective.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Khalidi U. A., Chaglassian T. H. The species distribution of xanthine oxidase. Biochem J. 1965 Oct;97(1):318–320. doi: 10.1042/bj0970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autor A. P., Frank L., Roberts R. J. Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatr Res. 1976 Mar;10(3):154–158. doi: 10.1203/00006450-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Betz A. L. Identification of hypoxanthine transport and xanthine oxidase activity in brain capillaries. J Neurochem. 1985 Feb;44(2):574–579. doi: 10.1111/j.1471-4159.1985.tb05451.x. [DOI] [PubMed] [Google Scholar]
- Boda D., Németh I., Hencz P., Dénes K. Effect of allopurinol treatment in premature infants with idiopathic respiratory distress syndrome. Dev Pharmacol Ther. 1984;7(6):357–367. doi: 10.1159/000457187. [DOI] [PubMed] [Google Scholar]
- Boros M., Karácsony G., Kaszaki J., Nagy S. Reperfusion mucosal damage after complete intestinal ischemia in the dog: the effects of antioxidant and phospholipase A2 inhibitor therapy. Surgery. 1993 Feb;113(2):184–191. [PubMed] [Google Scholar]
- Bratell S., Haraldsson G., Herlitz H., Jonsson O., Pettersson S., Scherstén T., Waldenström J. Protective effects of pretreatment with superoxide dismutase, catalase and oxypurinol on tubular damage caused by transient ischaemia. Acta Physiol Scand. 1990 Jul;139(3):417–425. doi: 10.1111/j.1748-1716.1990.tb08942.x. [DOI] [PubMed] [Google Scholar]
- Cho W. H., Kim D. G., Murase N., Mischinger H. J., Todo S., Starzl T. E. Comparison of superoxide dismutase, allopurinol, coenzyme Q10, and glutathione for the prevention of warm ischemic injury. Transplantation. 1990 Aug;50(2):353–355. [PMC free article] [PubMed] [Google Scholar]
- Cooke R. W. Early and late cranial ultrasonographic appearances and outcome in very low birthweight infants. Arch Dis Child. 1987 Sep;62(9):931–937. doi: 10.1136/adc.62.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D. K., Engelman R. M., Clement R., Otani H., Prasad M. R., Rao P. S. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun. 1987 Oct 14;148(1):314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz R. A., Ablow R. C., Warshaw J. B. Effect of vitamin E on the development of oxygen-induced lung injury in neonates. Ann N Y Acad Sci. 1982;393:452–466. doi: 10.1111/j.1749-6632.1982.tb31283.x. [DOI] [PubMed] [Google Scholar]
- Fish W. H., Cohen M., Franzek D., Williams J. M., Lemons J. A. Effect of intramuscular vitamin E on mortality and intracranial hemorrhage in neonates of 1000 grams or less. Pediatrics. 1990 Apr;85(4):578–584. [PubMed] [Google Scholar]
- Gerdin E., Tydén O., Eriksson U. J. The development of antioxidant enzymatic defense in the perinatal rat lung: activities of superoxide dismutase, glutathione peroxidase, and catalase. Pediatr Res. 1985 Jul;19(7):687–691. doi: 10.1203/00006450-198507000-00010. [DOI] [PubMed] [Google Scholar]
- Grune T., Siems W. G., Schneider W. Accumulation of aldehydic lipid peroxidation products during postanoxic reoxygenation of isolated rat hepatocytes. Free Radic Biol Med. 1993 Aug;15(2):125–132. doi: 10.1016/0891-5849(93)90051-u. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kawakami M., Yamauchi Y., Shimizu S., Nakamura M. Effect of allopurinol on ischemia and reperfusion-induced cerebral injury in spontaneously hypertensive rats. Stroke. 1986 Nov-Dec;17(6):1284–1287. doi: 10.1161/01.str.17.6.1284. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Jenkinson S. G., Roberts R. J., DeLemos R. A., Lawrence R. A., Coalson J. J., King R. J., Null D. M., Jr, Gerstmann D. R. Allopurinol-induced effects in premature baboons with respiratory distress syndrome. J Appl Physiol (1985) 1991 Mar;70(3):1160–1167. doi: 10.1152/jappl.1991.70.3.1160. [DOI] [PubMed] [Google Scholar]
- Malkiel S., Har-el R., Schwalb H., Uretzky G., Borman J. B., Chevion M. Interaction between allopurinol and copper: possible role in myocardial protection. Free Radic Res Commun. 1993;18(1):7–15. doi: 10.3109/10715769309149909. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mink R. B., Dutka A. J., Hallenbeck J. M. Allopurinol pretreatment improves evoked response recovery following global cerebral ischemia in dogs. Stroke. 1991 May;22(5):660–665. doi: 10.1161/01.str.22.5.660. [DOI] [PubMed] [Google Scholar]
- Palmer C., Vannucci R. C., Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res. 1990 Apr;27(4 Pt 1):332–336. doi: 10.1203/00006450-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Peachey N. S., Green D. J., Ripps H. Ocular ischemia and the effects of allopurinol on functional recovery in the retina of the arterially perfused cat eye. Invest Ophthalmol Vis Sci. 1993 Jan;34(1):58–65. [PubMed] [Google Scholar]
- Rosati R., Stortoni F., Filingeri V., Svegliati F., Cervelli V., Casciani C. U. Allopurinol and superoxide dismutase administration in prevention of rat kidney ischemic injury. Transplant Proc. 1988 Oct;20(5):928–930. [PubMed] [Google Scholar]
- Rosenfeld W., Evans H., Concepcion L., Jhaveri R., Schaeffer H., Friedman A. Prevention of bronchopulmonary dysplasia by administration of bovine superoxide dismutase in preterm infants with respiratory distress syndrome. J Pediatr. 1984 Nov;105(5):781–785. doi: 10.1016/s0022-3476(84)80307-8. [DOI] [PubMed] [Google Scholar]
- Russell G. A., Jeffers G., Cooke R. W. Plasma hypoxanthine: a marker for hypoxic-ischaemic induced periventricular leucomalacia? Arch Dis Child. 1992 Apr;67(4 Spec No):388–392. doi: 10.1136/adc.67.4_spec_no.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad O. D. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res. 1988 Feb;23(2):143–150. doi: 10.1203/00006450-198802000-00001. [DOI] [PubMed] [Google Scholar]
- Sinha S., Davies J., Toner N., Bogle S., Chiswick M. Vitamin E supplementation reduces frequency of periventricular haemorrhage in very preterm babies. Lancet. 1987 Feb 28;1(8531):466–471. doi: 10.1016/s0140-6736(87)92087-3. [DOI] [PubMed] [Google Scholar]
- Tan S., Radi R., Gaudier F., Evans R. A., Rivera A., Kirk K. A., Parks D. A. Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr Res. 1993 Sep;34(3):303–307. doi: 10.1203/00006450-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Tanswell A. K., Freeman B. A. Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. I. Developmental profiles. Pediatr Res. 1984 Jul;18(7):584–587. doi: 10.1203/00006450-198407000-00003. [DOI] [PubMed] [Google Scholar]
- Vettenranta K., Raivio K. O. Xanthine oxidase during human fetal development. Pediatr Res. 1990 Mar;27(3):286–288. doi: 10.1203/00006450-199003000-00017. [DOI] [PubMed] [Google Scholar]
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976 Apr;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]


