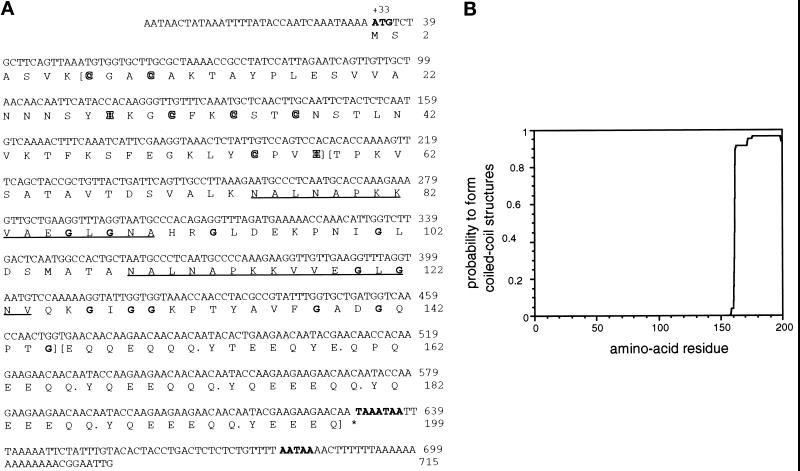
Figure 2.
(A) Nucleotide sequence and derived amino acid sequence of DdLim. The start codon, the stop codon, and two potential polyadenylation signals are indicated in bold letters in the nucleotide sequence. The protein sequence suggests the presence of three different structural domains; the borders of these domains are indicated by parentheses. The N-terminal LIM domain (amino residues 7–57), shows the defined spacing of cysteine and histidine residues (CX2CX17HX2 CX2CX2CX17CX2H) of the LIM domain (shown in outlined letters). The LIM domain is followed by a domain with a high proportion of glycine residues (indicated in bold). Within this domain a 16-amino acid motif is repeated twice (underlined) with two conserved alanine-to-valine exchanges. The high number of glycine residues in this domain is typical of the CRP/MLP group of LIM proteins. The C-terminal domain consists of a hepta-amino acid motif repeated eight times with minor deviations (indicated by a dot after each heptad). The DdLim sequence has been deposited in the GenBank with accession number U97699. (B) Analysis of DdLim sequence by the COILS algorithm of Lupas et al. (1991) predicts, with a high probability, that at least four hepta-repeats (repeat 4 to 7) will form a coiled-coil structure.