Figure 1. The structure of Hsp90 and its ATP-dependent molecular clamp.
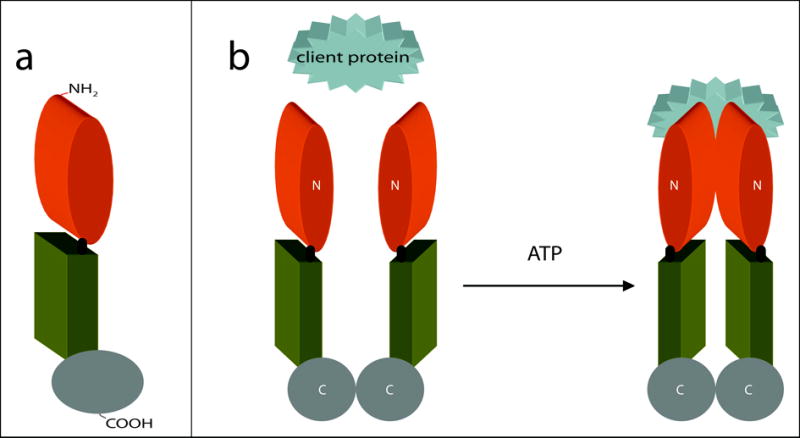
(a) Schematic representation of Hsp90. The Hsp90 monomer is comprised of three domains: the N-terminal domain responsible for ATP-binding (orange), a core domain (green), and a C-terminal domain that facilitates homodimerization (gray). In eukaryotes, a short charged region links the N-terminal and core domains (black). (b) ATP-driven molecular clamp cycle of Hsp90. In the absence of bound nucleotide, the C-termini (C) of two Hsp90 monomers interact to maintain an antiparallel dimer (left). Concurrently, the N-termini (N) of the Hsp90 homodimer preserve an open-state, facilitating the capture of client proteins (left). On the right, association with ATP induces modest changes in the conformation of Hsp90 that permit a transitory interaction between the opposing N-terminal domains. This produces the closed-form of Hsp90 where clamping of the substrate protein occurs.
