Figure 2. Hsp90α and Hsp90β enhance the solubility of proteins expressed in E. coli.
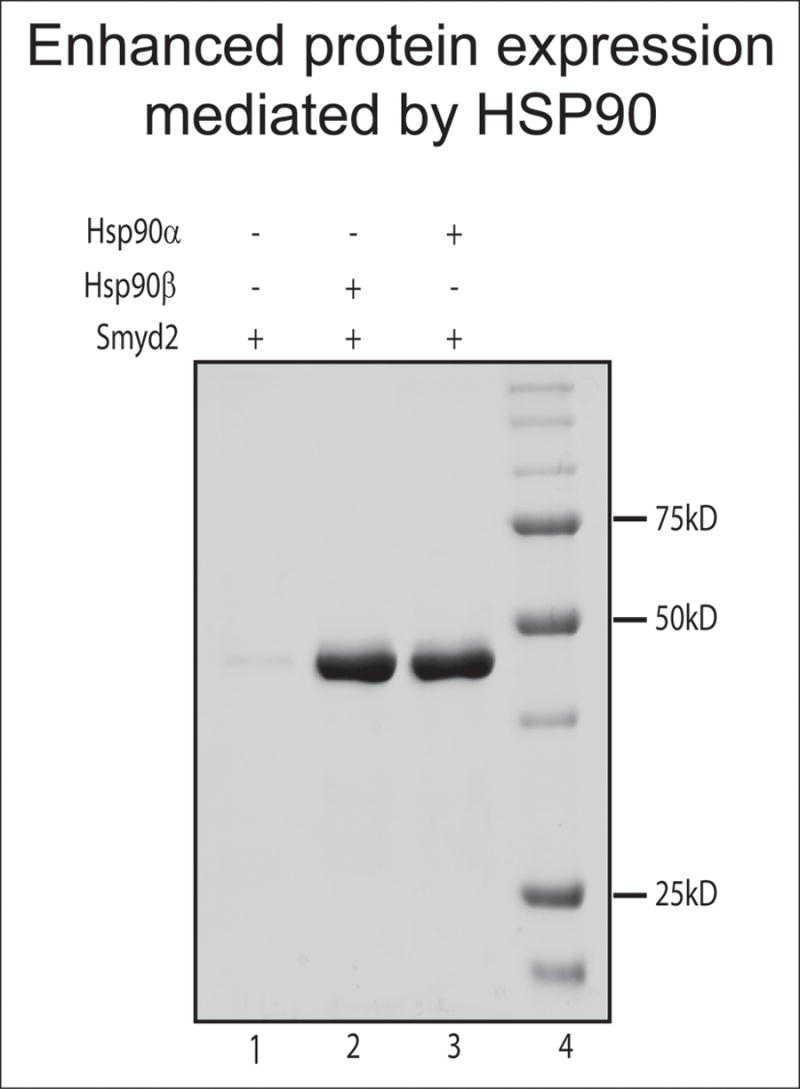
There are two isoforms of Hsp90 (Hsp90α and Hsp90β) which share 85% identity and maintain virtually indistinguishable functional properties [59]. Here, Hsp90 isoform-expressing Escherichia coli strains produce higher yields of soluble Smyd2. Wild type (lane1) and Hsp90 isoform-expressing (lanes 2 and 3) E. coli strains were transformed with a Smyd2 expression construct [45]. Equal amounts (2.5 g wet cell paste) of cultured E. coli cells were used for isolation and purification of Smyd2. The migration of molecular weight markers (in kiloDaltons) is indicated. The depicted results are representative of the enhanced solubility achieved with numerous proteins (Table 1) in Hsp90+ strains.
