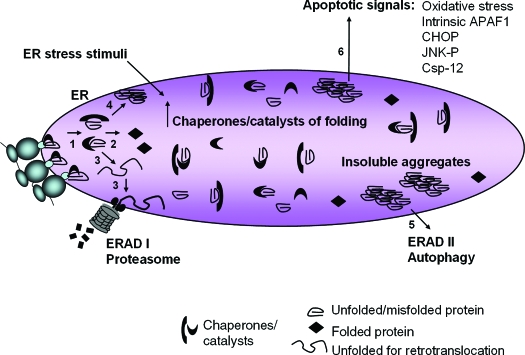
Figure 3.
Pathways of protein misfolding that lead to cell death. Nascent unfolded polypeptides enter the ER and interact with chaperones and catalysts of protein folding to mature into compact, thermodynamically favorable structures, (1) and (2). Failure of this process results in persistence of misfolded polypeptide-chaperone complexes (1) or extraction of soluble, misfolded protein from the ER and degradation through ERAD I (3). Formation of insoluble protein aggregates (4) requires clearance by autophagy (5). ER stress stimuli impair polypeptide folding and induce adaptive increases in chaperones and catalysts within the ER lumen through UPR sensor activation. Chronic or overwhelming stimuli elicit a number of apoptotic signals including oxidative stress, JNK activation, CHOP expression, cleavage of caspase 12, and activation of the intrinsic mitochondrial-dependent cell death pathway (6).