Figure 4.
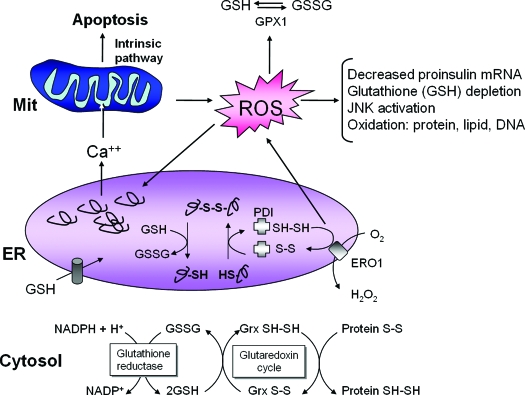
ER stress, protein misfolding, and oxidative stress are intimately interrelated. Protein folding within the ER lumen is ushered by a family of oxidoreductases that catalyze disulfide bond formation and isomerization. ER stress causes an increase in the formation of incorrect intermolecular and/or intramolecular disulfide bonds that require breakage and reformation for proteins to attain the appropriate folded conformation. PDI catalyzes disulfide bond formation and isomerization, whereas glutathione transported into the ER reduces improperly paired disulfide bonds. Reoxidation of PDI is mediated by ERO1; however, ROS are produced in the process. Cellular ROS can deplete glutathione and increase the misfolded protein load in the ER. In turn, ROS can also cause ER stress through modification of proteins and lipids that are necessary to maintain ER homeostasis. Consumption of excessive cellular glutathione due to ER stress could inhibit glutaredoxin reduction and cause accumulation of oxidized cytosolic proteins. ER stress also causes calcium leak from the ER for accumulation in the inner mitochondrial matrix. This calcium loading in the mitochondria can generate additional ROS through disruption of electron transport and opening of the mitochondrial permeability pore. Thus, accumulation of misfolded protein in the ER increases ROS production that can further amplify ER stress, disrupt insulin production, and cause cell death.