Abstract
Cardioviruses comprise a genus of picornaviruses that cause severe illnesses in rodents, but little is known about the prevalence, diversity, or spectrum of disease of such agents among humans. A single cardiovirus isolate, Saffold virus, was cultured in 1981 in stool from an infant with fever. Here, we describe the identification of a group of human cardioviruses that have been cloned directly from patient specimens, the first of which was detected using a pan-viral microarray in respiratory secretions from a child with influenza-like illness. Phylogenetic analysis of the nearly complete viral genome (7961 bp) revealed that this virus belongs to the Theiler's murine encephalomyelitis virus (TMEV) subgroup of cardioviruses and is most closely related to Saffold virus. Subsequent screening by RT-PCR of 719 additional respiratory specimens [637 (89%) from patients with acute respiratory illness] and 400 cerebrospinal fluid specimens from patients with neurological disease (aseptic meningitis, encephalitis, and multiple sclerosis) revealed no evidence of cardiovirus infection. However, screening of 751 stool specimens from 498 individuals in a gastroenteritis cohort resulted in the detection of 6 additional cardioviruses (1.2%). Although all 8 human cardioviruses (including Saffold virus) clustered together by phylogenetic analysis, significant sequence diversity was observed in the VP1 gene (66.9%–100% pairwise amino acid identities). These findings suggest that there exists a diverse group of novel human Theiler's murine encephalomyelitis virus-like cardioviruses that hitherto have gone largely undetected, are found primarily in the gastrointestinal tract, can be shed asymptomatically, and have potential links to enteric and extraintestinal disease.
Keywords: DNA microarrays, gastroenteritis, influenza-like illness, picornavirus, virus discovery
Picornaviruses are positive single-stranded RNA viruses that cause a variety of important disease states in humans and animals. Several genera of picornaviruses are recognized, based on genomic sequence and virus biology. The Cardiovirus genus of the family Picornaviridae consists of two subgroups: Theiler's murine encephalomyelitis virus (TMEV) and related viruses (Theiler-like virus NGS910 of rats, Vilyuisk virus) (1–3), and encephalomyocarditis virus (EMCV) and related viruses (EMCV, Mengovirus, Columbia SK virus, Maus–Elberfeld virus) (4). All these viruses infect rodents, replicate in the gastrointestinal (GI) tract and are transmitted by the fecal-oral route. Although enteric infection by these viruses is often mild or asymptomatic, extraintestinal spread of these viruses can occur and can lead to systemic disease (1). As their name implies, the EMCV-like agents cause encephalitis and myocarditis, whereas the TMEV family is linked to CNS infection. In experimental settings, intracerebral inoculation of mice with TMEV can produce acute encephalomyelitis and/or a chronic demyelinating disease resembling human multiple sclerosis (MS), depending upon the strain of TMEV used (5). Oral inoculation with TMEV may also result in encephalomyelitis, especially when large inocula are delivered to neonatal mice (6).
Whether authentic human cardioviruses exist has long been debated. The first candidate human cardiovirus was Vilyuisk virus, which was linked to Vilyuisk encephalitis, an unusual neurodegenerative disease found among the Yakuts people of Siberia in the 1950s and still endemic to the region (7, 8). The Vilyuisk virus was initially isolated from the cerebrospinal fluid (CSF) of an affected patient and underwent 41 serial passages in mice before sequencing and characterization as a TMEV-like picornavirus (3, 9). Given its sequence similarity to TMEV and its extensive passage history in mice, questions have arisen as to whether the virus may in fact be of murine origin. In 1981, another TMEV-related cardiovirus was cultured from the stool of an infant who presented with a febrile illness (10). Although early passages appeared to show that the virus was transmissible, long-term continuous propagation of the isolate has been problematic. The nearly complete genomic sequence of this isolate (provisionally called Saffold virus) was recovered from frozen stocks by cloning in 2007 and was found to be much more divergent from TMEV than Vilyuisk virus (10). However, neither Vilyuisk nor Saffold virus was cloned directly from primary clinical specimens, and the diversity, prevalence, and potential clinical manifestations of human cardiovirus infection have remained largely unexplored.
We have previously developed a pan-viral DNA microarray (Virochip; University of California, San Francisco) designed to detect known and novel viruses in clinical specimens on the basis of homology to conserved regions of known viral sequences (11). The current study uses microarrays from the third and fourth generations of this platform (Viro3, Viro4). The Viro3 platform has 19,841 viral oligonucleotides derived from all publicly available viral sequence as of June 2004 (12, 13). The Viro4 platform is a streamlined update of the Viro3 platform consisting of 14,740 viral oligonucleotides derived from all publicly available viral sequence as of June 2006. The Virochip has been used to detect novel pathogens such as the severe acute respiratory syndrome coronavirus (14) and XMRV, a retrovirus identified in prostate tissue of men with germ-line mutations in RNase L (15). The platform has also been successfully used to detect known and divergent viruses in acute respiratory tract infections in several recently published studies (12, 13, 16, 17).
In this study, we used the Virochip to screen respiratory secretions from patients with influenza-like illness who lacked a diagnosis despite extensive microbiological testing. In one such patient, we detected and fully sequenced a cardiovirus in the Saffold group. Related cardioviruses were subsequently found in stool specimens from an additional six individuals collected as part of a study examining household transmission of gastroenteritis (18). We report here the existence and overall phylogeny of a diverse group of human cardioviruses and discuss their potential association with human disease.
Results
Detection of a Cardiovirus in a Patient with Influenza-Like Illness.
A total of 460 respiratory secretions from patients meeting a case definition of influenza-like illness were screened for respiratory viruses by culture. In 108 culture-negative specimens selected from elderly and pediatric patients, 16 specimens remained negative after subsequent RT-PCR testing for respiratory syncytial virus (RSV), influenza A/B (Flu A/B), rhinovirus (RV), and enterovirus (EV). These 16 specimens were assayed for the presence of viruses using the Virochip (Viro3), with microarray analysis carried out using E-Predict and ranked z score analysis, as previously described (12, 19).
Four of the 16 specimens yielded a positive microarray hybridization signature suggestive of a virus. Two of the signatures corresponded to metapneumovirus, one signature corresponded to adenovirus, and one signature indicated the presence of a cardiovirus related to TMEV. From the microarray containing the cardiovirus signature, the highest intensity oligonucleotides mapped to the 5′-untranslated region (5′-UTR) and 2C gene of the TMEV genome, the most conserved regions among cardioviruses and picornaviruses in general (Fig. 1A, “ARRAY”). To recover viral sequence, we designed primers based on the highest intensity array features and alignment of well conserved sequences from four cardioviruses (TMEV-DA, TMEV-GDVII, Theiler-like NGS910 virus, and EMCV). One set of primers successfully amplified a 224-bp fragment from the viral 5′-UTR. The fragment shared 90% nucleotide identity with the 5′-UTR region of Theiler-like NGS910 virus. This finding established that the virus in question was indeed a cardiovirus and a relative of the TMEV group of viruses. We designated this initial cardiovirus strain UC1.
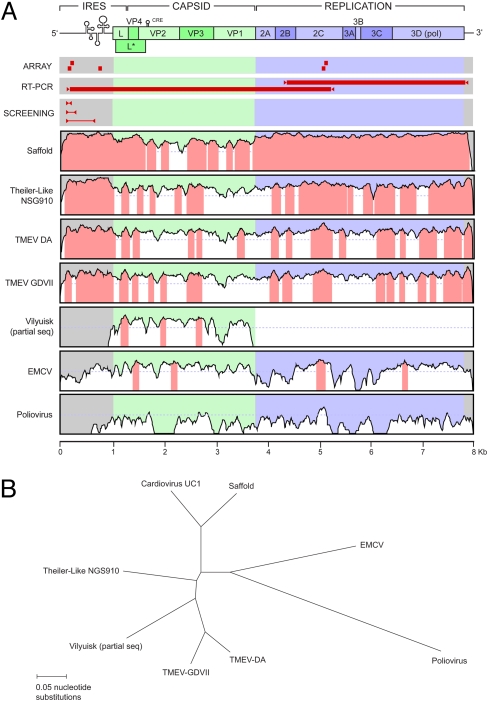
Fig. 1.
Genome sequence of UC1. (A) Genome sequence similarity plots compare UC1 with Saffold virus, Theiler-Like NGS910 virus, TMEV-DA, TMEV-GDVII, Vilyuisk virus (partial sequence only), EMCV, and poliovirus. The y axis scale for each plot represents percentage of nucleotide identities from 0% to 100%. Regions of the genome with percentage of nucleotide identities of >70% are highlighted in pink. The Virochip oligonucleotides used to detect UC1 (“ARRAY”), the fragments generated by long-range RT-PCR and used to sequence most of the virus (“RT-PCR”), and the cardiovirus primers and resulting PCR fragments used for screening of stool, CSF, and respiratory secretions (“SCREENING”) are also shown mapped onto the UC1 genome. The sequences of these primers are provided in Table S1. (B) Radial tree depicts the phylogenetic relationships between the genomes of UC1 and the seven aforementioned cardioviruses.
Complete Genome Sequencing and Analysis of UC1.
To clone and sequence the remainder of the UC1 genome, additional short fragments were first obtained from conserved regions in the 2C (helicase) and 3D (polymerase) genes by use of consensus PCR primers derived from alignment of the four cardioviruses mentioned previously. Long-range RT-PCR using specific primers was then used to bridge the gaps. This resulted in PCR amplification of two long overlapping fragments (≈5.3 and 3.7 kB in size) jointly spanning nearly the entire length of the virus genome (Fig. 1A, “RT-PCR”). Cloned ends of the genome were recovered and sequenced using a RACE amplification protocol (20, 21).
The nearly complete sequence of UC1 is 7961 nt in length and forms a distinct branch in the Cardiovirus genus with Saffold virus (Fig. 1B). The overall nucleotide identity to Saffold virus is >90% in the 5′-UTR and the region coding for the nonstructural proteins but only 70% in the region coding for the capsid proteins (Fig. 1A, “Saffold”). There is much less overall nucleotide sequence identity to other members of the TMEV subgroup (70–80%) and EMCV (50–55%). A poly(C) tract that has been reported in EMCV but not in TMEV strains is not present in the 5′-UTR of UC1. Similar to other cardioviruses, the ORF of UC1 is predicted to code for a single 2296-amino acid polyprotein that is subsequently cleaved into the L protein, the capsid proteins (VP1, VP2, VP3, and VP4), and nonstructural proteins involved in viral replication (2A, 2B, 2C, 3A, 3B, 3C, and 3D) (Fig. 1A). Like Saffold virus, UC1 encodes an L protein containing a zinc finger, an acidic domain, and a partially deleted Ser/Thr-rich domain (22, 23) and potentially encodes a severely truncated L* protein that begins with an ACG codon rather than AUG (22, 23) [supporting information (SI) Fig. S1A].
In cardioviruses, the surface loops CD of VP1 and EF of VP2 are exposed on the capsid surface and are thought to be involved in host cell tropism and viral pathogenesis (24). These loops are the regions of greatest divergence between UC1 and the other cardioviruses, including Saffold virus (Fig. S1B). Between UC1 and Saffold virus, there is 52% and 61% amino acid identity in the exposed surface loops CD and EF, respectively. The corresponding identities (29% and 24%) are much lower between UC1 and the rodent cardioviruses.
Comparison of UC1 Amino Acid Sequence with Other Cardioviruses.
The level of divergence between the sequence of UC1 and other cardioviruses is maintained at the amino acid level. Between UC1 and Saffold virus, the capsid proteins VP1, VP2, and VP3 are only 77–85% identical, whereas the nonstructural proteins are highly conserved (98% overall identity) (Table 1). The amino acid identities between UC1 and its closest rodent relatives (NGS910 virus and TMEV) are much lower, 56–80% for the capsid proteins and 83–91% for the nonstructural proteins. These comparisons confirm that UC1 is most closely related to Saffold virus, although there is significant sequence divergence in the capsid proteins containing the putative receptor binding sites.
Table 1.
Amino acid identity of predicted UC1 proteins
| Gene | Predicted size, aa | Percent amino acid identity to |
|||||
|---|---|---|---|---|---|---|---|
| Saffold | NGS910 | Vilyuisk | TMEV-DA | EMCV | Polio | ||
| L protein | 71 | 77 | 61 | 60 | 60 | 26 | 0 |
| VP4 | 72 | 99 | 72 | 72 | 68 | 62 | 19 |
| VP2 | 269 | 83 | 69 | 67 | 71 | 64 | 30 |
| VP3 | 231 | 85 | 80 | 76 | 75 | 68 | 28 |
| VP1 | 275 | 77 | 56 | 55 | 59 | 48 | 14 |
| Nonstructural | 1389 | 98 | 91 | 83 | 40 | 22 | |
| Polyprotein | 2296 | 91 | 76 | 71 | 52 | 22 | |
Prevalence of Cardioviruses in Clinical Specimens.
To investigate the prevalence of cardiovirus infection in acute human illnesses, we designed PCR primers targeting the 5′-UTR to amplify cardioviruses by real-time one-step RT-PCR. In our initial screen, we ran two RT-PCRs using conserved primers designed to amplify 102-bp and 224-bp fragments from the 5′-UTR of UC1, Saffold virus, or all mouse strains of TMEV. By probit analysis (i.e., the concentration of the target sequence testing positive in 95% of cases) using in vitro transcribed UC1 mRNA, the sensitivity of the RT-PCR assay for detection of cardioviruses was 600 copies. Standard curves generated using pooled cardiovirus-negative specimens spiked with UC1 mRNA were linear from 104 to 1011 copies/ml (R2 = 0.9831–0.9944, Fig. S2). The presence of PCR inhibitors was estimated to be <3% by yeast RNA spiking experiments on randomly selected stool specimens (only 2 of 95 RT-PCRs failed to amplify the yeast positive control). All positives in the initial screen were sequenced and then further confirmed by another RT-PCR using primers designed to amplify an overlapping 608-bp fragment (Fig. 1A, “SCREENING”).
Since UC1 was first identified in respiratory secretions, we screened 719 respiratory specimens from two large groups of patients: 278 nasopharyngeal aspirates from pediatric patients at a single hospital (190 specimens from patients with an acute respiratory illness) (13) and 441 pooled oropharyngeal and nasopharyngeal swabs from individuals in California with influenza-like illness (25). None of the 719 total respiratory specimens tested was positive for cardioviruses.
We next conducted screening of CSF specimens from patients with aseptic meningitis (n = 60), patients with encephalitis (n = 300), and patients with MS (n = 40) for cardioviruses by RT-PCR. None of the 400 CSF specimens tested was found to be positive.
Given the prominent association of picornaviruses with enteric infection and the known fecal-oral route of transmission, we then sought to assess the prevalence of human cardioviruses in stool. We examined 751 stool specimens from 498 individuals collected as part of a cohort study of household transmission of Helicobacter pylori and gastroenteritis (18). The vast majority of subjects were children, with 443 (89%) children younger than 5 years, 30 (6%) children between 5 and 18 years, and 25 (5%) adults. Specimens from 6 children (1.2% of the 498 individuals) were positive for cardioviruses (strains UC2–UC7). All cardiovirus-positive stool specimens were from children <2 years old and from different households. Symptoms in the 6 children included diarrhea and vomiting in 3 (50%) and diarrhea only in 1 (17%); the remaining 2 children were asymptomatic. Of note, from 2 of the symptomatic children, one with diarrhea and vomiting and the other with diarrhea, a cardiovirus was identified not during acute illness but in a specimen obtained months after each child had recovered.
To investigate the possibility of coinfection with additional viruses, we used the Virochip (Viro4) to analyze the nine available specimens collected from the six cardiovirus-positive cases (Table 2). As expected, all six cardiovirus-positive cases were positive for a cardiovirus by Virochip. In three of the cardiovirus-positive stool specimens, there was evidence of coinfection: in two specimens by caliciviruses (norovirus and sapovirus) and in one specimen by a rotavirus. In the other three individuals, viruses other than cardioviruses were detected in the stool at the time of the first visit (adenovirus, norovirus and parechovirus, norovirus and enterovirus), but only cardiovirus was detected in the stool by the second visit. All Virochip results were subsequently confirmed by PCR and sequencing using virus-specific primers.
Table 2.
Patients with stool positive for cardioviruses
| ID | Age at first visit, months | Number ill in household | Days between visits 1 and 2 | Clinical symptoms |
Virochip/PCR results |
||
|---|---|---|---|---|---|---|---|
| 10 days prior to visit 1 | Between visits 1 and 2 | Visit 1 | Visit 2 | ||||
| UC2 | 8.4 | 4/10 | — | Diarrhea/vomiting | — | Cardiovirus, rotavirus | — |
| UC3 | 6.1 | 1/5 | 139 | None | none | — | Cardiovirus, norovirus |
| UC4 | 21.4 | 1/9 | 91 | None | none | Adenovirus | Cardiovirus |
| UC5 | 16.3 | 6/6 | 95 | Diarrhea/vomiting | none | Norovirus, parechovirus | Cardiovirus |
| UC6 | 14.0 | 1/5 | — | Diarrhea/vomiting | — | Cardiovirus, sapovirus | — |
| UC7 | 18.6 | 3/7 | 94 | Diarrhea | none | Norovirus, enterovirus | Cardiovirus |
Dashes indicate entries for which data and/or specimens were not available.
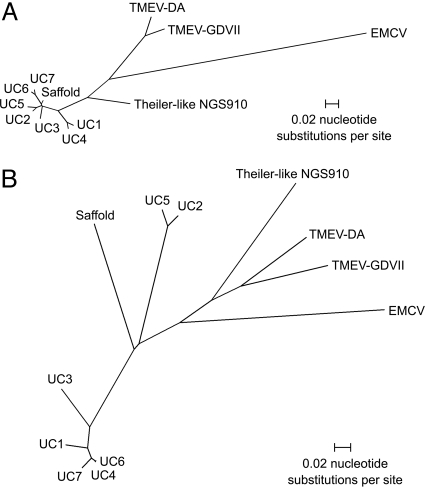
To assess the sequence variation within different cardiovirus strains, we analyzed a 608-bp region from the 5′-UTR and an 819-bp region corresponding to the VP1 gene for the six positive cardiovirus cases (Fig. 2). The sequence variations within the 608-bp region from the 5′-UTR (2.0–9.1%) and within the 819-bp region corresponding to the VP1 gene (0.3–36.7%) were consistent with infection by independently acquired cardiovirus strains. The amino acid sequence identities in the VP1 gene were lowest between UC2/UC5 and the other cardioviruses (66.9% for Saffold virus, 71.0–72.8% for the other UC strains).
Fig. 2.
Strain variation of human cardioviruses. (A) Radial tree of a 608-bp region within the 5′-UTR. (B) Radial tree of an 819-bp region corresponding to the VP1 gene. Strain designations UC2 to UC7 correspond to patients as listed in Table 2.
Discussion
Using a pan-viral microarray, we analyzed 16 respiratory specimens from patients with influenza-like illness who still lacked a diagnosis after extensive tests for respiratory viruses. In one specimen, we found a signature for a cardiovirus. Sequence recovery of the genome and phylogenetic analysis revealed that this virus (UC1) is divergent from the rodent cardioviruses and clusters with the Saffold agent. Like Saffold virus, UC1 may code for a truncated L* protein (Fig. S1A) that has been implicated in viral persistence and chronic infection of the CNS in TMEV (26). However, because the L* protein of UC1 begins with an ACG codon rather than AUG, it is unclear whether any functional protein is actually expressed, although small amounts of L* protein have been detected in TMEV strains carrying the ACG codon (27).
The binding of sialic acid to TMEV is strongly associated with persistence and neurovirulence, and three amino acids in the VP2 protein are directly involved in this interaction (28, 29). In both UC1 and Saffold virus, there is a substitution or deletion at each of these three positions (Fig. S1B), suggesting that sialic acid is unlikely to serve as a receptor for these viruses. Although the cellular receptor is presently unknown, the sequences of UC1 and Saffold virus are most divergent in the capsid region, sharing only 77% and 83% amino acid identity in the VP1 and VP2 proteins, respectively, and 52% and 61% identity in the exposed surface loops CD and EF, respectively. These differences may reflect the use of distinct cellular receptors or may be the result of immune selection during virus evolution (or both); further studies will be required to shed light on these issues.
Cardioviruses were detected in six children out of a total of 498 individuals (1.2%) enrolled in a large gastroenteritis study. Although the initial specimen that was used to culture Saffold virus was collected >25 years ago, cardioviruses UC1 through UC7 were collected from 2000 to 2006, indicating that human cardioviruses continue to circulate in the population. Despite the use of screening RT-PCR assays able to detect all strains of TMEV, cardioviruses detected in human clinical specimens clustered together and were phylogenetically distinct from the rodent cardioviruses (Fig. 2).
Further studies will be required to define the pathogenic role of cardiovirus infection in the intestine fully. Although we did recover a cardiovirus from a number of cases with symptomatic enteritis, other potential GI pathogens were also detected in these cases. Thus, it is presently unclear how frequently enteric cardioviral infection produces clinical illness. Moreover, we detected cardioviruses in stool from subjects without enteritis, suggesting that asymptomatic shedding of cardioviruses in the GI tract can and does occur. In this respect, cardiovirus infection in humans may mimic that of murine TMEV, which is often shed asymptomatically in naturally acquired infections (30).
Cardiovirus infection outside the GI tract is sometimes associated with severe disease in rodents, including encephalomyelitis, demyelinating disease, and myocarditis (1), although only a small percentage of mice naturally infected with TMEV develop systemic disease (1, 5). Our wider screening for cardioviruses indicates that cardiovirus infection is uncommon in the setting of acute respiratory or neurological disease (e.g., aseptic meningitis, encephalitis, MS). However, while this manuscript was under review, Abed and Boivin (31) reported detection of Saffold-like cardioviruses in three children with acute respiratory illness. Moreover, in a case of influenza-like illness reported here, a cardiovirus was the sole agent identified despite comprehensive testing with culture, PCR, and a pan-viral microarray, suggesting that cardioviruses may be pathogenic outside the GI tract in at least some instances.
One remarkable finding from this study was the diversity of the human cardioviruses that have been identified. For the family Picornaviridae, the definition of a new species in a genus is having <70% amino acid identity in the coding regions of either VP1, 2C, 3C, or 3D (32). By this strict definition, cardioviruses UC2 and UC5 would classify as a novel species distinct from Saffold virus, with 66.9% amino acid identity in the VP1 gene. However, since cardioviruses UC1 through UC7 and Saffold virus as a whole clearly define a separate group within the Cardiovirus genus by phylogenetic analysis (Figs. 1B and 2), we propose a systematic nomenclature for the human cardioviruses, designating all members of this group HTCV, for human TMEV-like cardiovirus, and referring to the strains in this group by a brief suffix (e.g., Saffold agent would be designated HTCV-Saf, UC1 would be designated HTCV-UC1).
Several lines of evidence support the inference that HTCVs are bona fide human viruses and not the products of sporadic viral cross-over events from rodents to humans: (i) all seven cardioviruses from humans in this study are strains of HTCV, with no mouse TMEV sequences detected in 1870 total clinical specimens despite screening using two consensus PCR primer sets designed to amplify UC1, Saffold virus, or mouse TMEV; (ii) sequence variations within HTCV UC1–7 are most consistent with independent acquisition of different virus strains by patients; and (iii) HTCV is substantially diverged from the rodent cardioviruses, especially in the capsid region containing the putative receptor binding sites. Taken together, our findings indicate that HTCVs are novel human picornaviruses in the Cardiovirus genus that are found primarily in the GI tract, can be shed asymptomatically, and have potential links to self-limited enteric disease and, rarely, to influenza-like illness. Although the full spectrum of clinical diseases linked to HTCV and the mechanisms underlying viral replication remain to be elucidated, the studies reported here now open all these questions to direct experimental scrutiny.
Materials and Methods
Clinical Specimens.
Respiratory secretions from the California Influenza Surveillance Program study.
A total of 943 respiratory specimens were sent to the California Department of Health Services (DHS) during the 2005–2006 season (25). Among these 943 specimens, 460 were pooled nasopharyngeal and oropharyngeal swabs collected as part of the California Influenza Surveillance Program (CISP) study under protocols approved by the DHS. Patients enrolled in the CISP study fulfilled a clinical case definition of influenza-like illness (temperature of 37.8°C or greater and a cough and/or sore throat in the absence of a known cause other than influenza). Sixty percent, or 280 specimens, were positive for a virus by culture. Among the remaining 180 culture-negative specimens, a subset of 108 specimens selected from elderly and pediatric patients was then subjected to further screening by RT-PCR to exclude cases of RSV, Flu A/B, RV, and EV (33). Sixteen specimens negative by culture and RT-PCR were then examined using the Virochip. We subsequently screened 441 CISP specimens with remaining available specimen material (96% of the 460 total collected specimens) for cardioviruses by RT-PCR.
Respiratory secretions from the UCSF pediatric respiratory infections study.
This collection consisted of 278 consecutive nasopharyngeal aspirates from pediatric patients seen at UCSF from December 2003 to June 2004 (13). All specimens were collected under protocols approved by the UCSF Institutional Review Board. In this group, 190 of the patients (68%) had a respiratory illness, defined as an upper respiratory infection, bronchiolitis, croup, asthma exacerbation, or pneumonia. The remaining 88 patients (32%) were asymptomatic.
Stool from the Stanford Infection and Family Transmission cohort.
The Stanford Infection and Family Transmission (SIFT) cohort of 4333 individuals was initiated in 1999 to evaluate the association between H. pylori infection and gastroenteritis transmission prospectively (18). Among the 3063 subjects who consented to further use of biological specimens, 774 stool specimens were obtained from 514 individuals; of those, 751 specimens from 498 subjects were available for study. Additional details on the 751 specimens screened for cardioviruses by RT-PCR are described in SI Text.
CSF specimens from patients with aseptic meningitis, encephalitis, and MS.
A total of 60 CSF specimens from patients with clinically diagnosed aseptic meningitis, 300 CSF specimens from patients with encephalitis (who lacked a diagnosis despite comprehensive testing) (34), and 40 CSF specimens from patients with MS were screened for cardioviruses by RT-PCR. Specimens were collected under protocols approved by the California DHS (encephalitis specimens) or the UCSF Institutional Review Board (aseptic meningitis and MS specimens).
Specimen Preparation and Diagnostic Testing.
In the CISP study, routine tube culture or shell vial culture of pooled nasopharyngeal and oropharyngeal swab specimens followed by specific monoclonal antibody testing for viral identification was performed as previously described (33, 35). Total nucleic acid was then extracted from the specimens using the MasterPure Complete DNA and RNA Purification Kit (Epicentre). Real-time one-step RT-PCR assays for RSV, FluA/B, and picornavirus (inclusive of RV and EV) were then performed as previously described (25, 33, 36). In the UCSF pediatric respiratory infections study, 200-μl aliquots of nasopharyngeal lavage were used to extract RNA using the RNeasy Mini Kit (Qiagen Corporation), including on-column DNase digestion. In the SIFT cohort, stool was suspended in 2 ml of PBS at 10% weight per volume and the PureLink96 Viral RNA/DNA Kit (Invitrogen) was used to extract RNA for RT-PCR and Virochip analysis. Cerebrospinal fluid specimens were processed using either a Zymo MiniRNA Isolation Kit (Zymo Research) or the MasterPure Complete DNA and RNA Purification Kit.
Virochip analysis of CISP and SIFT specimens was carried out as previously described (14). Extracted nucleic acid specimens were amplified and labeled using a Round A/B protocol and were hybridized to the Virochip. Microarrays (National Center for Biotechnology Information GEO platforms GPL3429 for Viro3 and GPL6862 for Viro4) were scanned with an Axon 4000B scanner (Axon Instruments). Virochip results were analyzed using cluster analysis, E-Predict, and z score analysis as previously described (12, 19, 37). All Virochip microarrays have been submitted to the GEO database (National Center for Biotechnology Information GEO series number GSE11569, accession numbers GSM291246–GSM291254).
Complete Genome Cloning and Sequencing (UC1 strain).
Conserved primers from the 5′UTR of cardioviruses were designed based on the highest intensity microarray oligonucleotides and alignment of well conserved sequences from four cardioviruses for which full genome sequences were available: TMEV-DA, TMEV-GDVII, Theiler-like NGS910 virus, and EMCV. After short viral fragments were obtained, six sets of specific primers derived from sequenced fragments and conserved primers were then used to sequence the genome by long-range RT-PCR and 5′/3′ RACE (rapid amplification of cDNA ends). Amplicons for sequencing were cloned into plasmid vectors using the TOPO TA Cloning System (Invitrogen) and sequenced on an ABI3130 Genetic Analyzer (Applied Biosystems) using standard Big Dye terminator (version 3.1) sequencing chemistry. The completed genome sequence of UC1 has been deposited into GenBank (GenBank accession number EU376394).
Phylogenetic Analysis (UC1 strain).
Nucleotide and protein sequences associated with the following reference virus genomes were obtained from GenBank: Saffold virus (NC_009448), TMEV-DA (M20301), TMEV-GDVII (NC_001366), Theiler-like NGS910 virus (AB090161), EMCV (NC_001479), poliovirus (NC_002048), and the partially sequenced genome of Vilyuisk virus (M94868). For amino acid analysis, ORFs predicted using ORF Finder (National Center for Biotechnology Information) were used. Multiple sequence alignment was performed using ClustalX (version 1.83). Neighbor-joining trees using the Kimura two-parameter distance correction were generated using 1000 bootstrap replicates and displayed using MEGA (version 3.1). Sequence identities were calculated using BioEdit (version 7.0.9.0).
RT-PCR Screening for Cardioviruses.
Real-time quantitative RT-PCR (qRT-PCR) screening for cardioviruses with SYBR Green I (Invitrogen) was performed using conserved PCR primer sets CardioUTR-1F/CardioUTR-2R-A and CardioUTR-1F/CardioUTR-2R-B (Table S1) on a DNA Engine Opticon System (Bio-Rad). To determine limits of sensitivity of the qRT-PCR assay, probit analysis of results from 10 qRT-PCR replicates of eight serial half-log dilutions of in vitro transcribed UC1 mRNA (from a starting concentration of ∼105 copies/ml) was performed using StatsDirect (StatsDirect Ltd.). Standard curves of the qRT-PCR assay were calculated from 3 qRT-PCR replicates of seven serial log dilutions of RNA extracted from pooled respiratory secretions, stool suspensions, and PBS spiked with UC1 RNA (10 specimens per pool). To assess for the presence of PCR inhibitors, RT-PCR for yeast was carried out on 95 randomly selected stool samples, each spiked with 1 ng of in vitro transcribed Saccharomyces cerevisiae intergenic RNA as a positive control (38).
Positive bands corresponding to the expected 102-bp and 224-bp amplicons were cloned and sequenced in both directions using vector primers M13F and M13R. Secondary confirmation of all positive reactions was performed using RT-PCR with primers CardioUTR-1F and CardioUTR-3R (Table S1), which generated a larger 608-bp amplicon, also in the 5′-UTR. To obtain the full sequences of the VP1 gene in strains UC2 through UC7, RT-PCRs were performed using conserved primers flanking the VP1 region of UC1 and Saffold virus (Table S1). The sequences of the 5′-UTR and VP1 amplicons corresponding to cardiovirus strains UC2 through UC7 have been deposited in GenBank (accession numbers EU604739–EU604750).
PCR Confirmation for Virochip-Positive Stool Specimens.
All nine specimens collected from the six positive cardiovirus cases were analyzed using the Virochip as previously described (11, 12). Confirmatory PCR for calicivirus, adenovirus, and parechovirus was carried out using conserved primers as previously reported (39–41). Amplified PCR bands of the expected size were gel extracted and sequenced using standard BigDye chemistry on an ABI3130 (Applied Biosystems).
Supplementary Material
Acknowledgments.
We thank Silvi Rouskin for expert technical assistance and Amy Kistler, Patrick Tang, Anatoly Urisman, and Yiyang Xu for helpful suggestions on the manuscript. We thank Drs. Stephen Hauser and Jorge Oksenberg for generously providing CSF specimens from patients with MS for cardiovirus screening. These studies were supported by grants from the Doris Duke Charitable Foundation (to J.L.D. and D.G.), Howard Hughes Medical Institute (to J.L.D. and D.G.), and Packard Foundation (to J.L.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805968105/DCSupplemental.
References
- 1.Brahic M, Bureau JF, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu Rev Microbiol. 2005;59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- 2.Ohsawa K, Watanabe Y, Miyata H, Sato H. Genetic analysis of a Theiler-like virus isolated from rats. Comp Med. 2003;53:191–196. [PubMed] [Google Scholar]
- 3.Pritchard AE, Strom T, Lipton HL. Nucleotide sequence identifies Vilyuisk virus as a divergent Theiler's virus. Virology. 1992;191:469–472. doi: 10.1016/0042-6822(92)90212-8. [DOI] [PubMed] [Google Scholar]
- 4.Duke GM, Hoffman MA, Palmenberg AC. Sequence and structural elements that contribute to efficient encephalomyocarditis virus RNA translation. J Virol. 1992;66:1602–1609. doi: 10.1128/jvi.66.3.1602-1609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler's virus infection: A model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha-Lee YM, et al. Mode of spread to and within the central nervous system after oral infection of neonatal mice with the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1995;69:7354–7361. doi: 10.1128/jvi.69.11.7354-7361.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrov PA. V. Vilyuisk encephalitis in the Yakut Republic (U.S.S. R) Am J Trop Med Hyg. 1970;19:146–150. doi: 10.4269/ajtmh.1970.19.146. [DOI] [PubMed] [Google Scholar]
- 8.Vladimirtsev VA, et al. Family clustering of Viliuisk encephalomyelitis in traditional and new geographic regions. Emerg Infect Dis. 2007;13:1321–1326. doi: 10.3201/eid1309.061585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipton HL, Friedmann A, Sethi P, Crowther JR. Characterization of Vilyuisk virus as a picornavirus. J Med Virol. 1983;12:195–203. doi: 10.1002/jmv.1890120305. [DOI] [PubMed] [Google Scholar]
- 10.Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol. 2007;45:2144–2150. doi: 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, et al. Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci USA. 2002;99:15687–15692. doi: 10.1073/pnas.242579699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu CY, et al. Microarray detection of human parainfluenzavirus 4 infection associated with respiratory failure in an immunocompetent adult. Clin Infect Dis. 2006;43:e71–e76. doi: 10.1086/507896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu CY, et al. Utility of DNA microarrays for detection of viruses in pediatric acute respiratory infections. J Pediatr. 2008;153:76–83. doi: 10.1016/j.jpeds.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, et al. Viral discovery and sequence recovery using DNA microarrays. PLoS Biol. 2003;1:E2. doi: 10.1371/journal.pbio.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urisman A, et al. Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:E25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Chiu CY, et al. Diagnosis of a critical respiratory illness caused by human metapneumovirus by use of a pan-virus microarray. J Clin Microbiol. 2007;45:2340–2343. doi: 10.1128/JCM.00364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kistler A, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry S, de la Luz Sanchez M, Hurst PK, Parsonnet J. Household transmission of gastroenteritis. Emerg Infect Dis. 2005;11:1093–1096. doi: 10.3201/eid1107.040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urisman A, et al. E-Predict: A computational strategy for species identification based on observed DNA microarray hybridization patterns. Genome Biol. 2005;6:R78. doi: 10.1186/gb-2005-6-9-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scotto-Lavino E, Du G, Frohman MA. 3′ end cDNA amplification using classic RACE. Nat Protoc. 2006;1:2742–2745. doi: 10.1038/nprot.2006.481. [DOI] [PubMed] [Google Scholar]
- 21.Scotto-Lavino E, Du G, Frohman MA. 5′ end cDNA amplification using classic RACE. Nat Protoc. 2006;1:2555–2562. doi: 10.1038/nprot.2006.480. [DOI] [PubMed] [Google Scholar]
- 22.Kong WP, Ghadge GD, Roos RP. Involvement of cardiovirus leader in host cell-restricted virus expression. Proc Natl Acad Sci USA. 1994;91:1796–1800. doi: 10.1073/pnas.91.5.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong WP, Roos RP. Alternative translation initiation site in the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1991;65:3395–3399. doi: 10.1128/jvi.65.6.3395-3399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jnaoui K, Michiels T. Adaptation of Theiler's virus to L929 cells: Mutations in the putative receptor binding site on the capsid map to neutralization sites and modulate viral persistence. Virology. 1998;244:397–404. doi: 10.1006/viro.1998.9134. [DOI] [PubMed] [Google Scholar]
- 25.Louie JK, et al. Creating a model program for influenza surveillance in California: Results from the 2005–2006 influenza season. Am J Prev Med. 2007;33:353–357. doi: 10.1016/j.amepre.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Ghadge GD, Ma L, Sato S, Kim J, Roos RP. A protein critical for a Theiler's virus-induced immune system-mediated demyelinating disease has a cell type-specific antiapoptotic effect and a key role in virus persistence. J Virol. 1998;72:8605–8612. doi: 10.1128/jvi.72.11.8605-8612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Eyll O, Michiels T. Non-AUG-initiated internal translation of the L* protein of Theiler's virus and importance of this protein for viral persistence. J Virol. 2002;76:10665–10673. doi: 10.1128/JVI.76.21.10665-10673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar AS, Kallio P, Luo M, Lipton HL. Amino acid substitutions in VP2 residues contacting sialic acid in low-neurovirulence BeAn virus dramatically reduce viral binding and spread of infection. J Virol. 2003;77:2709–2716. doi: 10.1128/JVI.77.4.2709-2716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Luo Y, Wu Y, Tsao J, Luo M. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J Virol. 2000;74:1477–1485. doi: 10.1128/jvi.74.3.1477-1485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownstein D, Bhatt P, Ardito R, Paturzo F, Johnson E. Duration and patterns of transmission of Theiler's mouse encephalomyelitis virus infection. Lab Anim Sci. 1989;39:299–301. [PubMed] [Google Scholar]
- 31.Abed Y, Boivin G. New saffold cardioviruses in 3 children, Canada. Emerg Infect Dis. 2008;14:834–836. doi: 10.3201/eid1405.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fauquet CM, Mayo MA, Maniloff J. Virus Taxonomy, Classification, and Nomenclature of Viruses. San Diego, CA: Elsevier Academic; 2005. [Google Scholar]
- 33.Louie JK, et al. Characterization of viral agents causing acute respiratory infection in a San Francisco University Medical Center Clinic during the influenza season. Clin Infect Dis. 2005;41:822–828. doi: 10.1086/432800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glaser CA, et al. Beyond viruses: Clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–1577. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 35.Louie JK, et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41:262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kares S, et al. Real-time PCR for rapid diagnosis of entero- and rhinovirus infections using LightCycler. J Clin Virol. 2004;29:99–104. doi: 10.1016/s1386-6532(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 37.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shock JL, Fischer KF, DeRisi JL. Whole-genome analysis of mRNA decay in Plasmodium falciparum reveals a global lengthening of mRNA half-life during the intra-erythrocytic development cycle. Genome Biol. 2007;8:R134. doi: 10.1186/gb-2007-8-7-r134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echavarria M, Forman M, Ticehurst J, Dumler JS, Charache P. PCR method for detection of adenovirus in urine of healthy and human immunodeficiency virus-infected individuals. J Clin Microbiol. 1998;36:3323–3326. doi: 10.1128/jcm.36.11.3323-3326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farkas T, et al. Genetic diversity among sapoviruses. Arch Virol. 2004;149:1309–1323. doi: 10.1007/s00705-004-0296-9. [DOI] [PubMed] [Google Scholar]
- 41.Legay V, Chomel JJ, Lina B. Specific RT-PCR procedure for the detection of human parechovirus type 1 genome in clinical samples. J Virol Methods. 2002;102:157–160. doi: 10.1016/s0166-0934(01)00435-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.