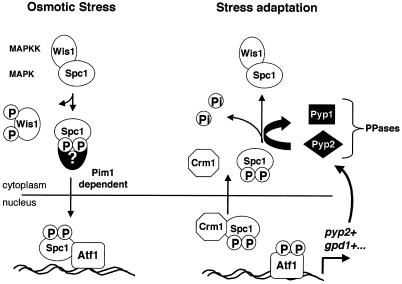
Figure 9.
Model for the nucleocytoplasmic transport of the SAPK Spc1/StyI in fission yeast. Osmotic stress results in the phosphorylation of the MAPKK Wis1, which in turn phosphorylates Spc1, releasing it from the cytoplasmic anchoring complex. Phosphorylated Spc1 is translocated into the nucleus via association with an unknown nuclear import protein. The import is dependent on the Ran exchange factor Pim1. Activated Spc1 released in the nucleus displays strong affinity for its substrate and nuclear anchor, the transcription factor Atf1 (Gaits et al., 1998). Spc1 activates Atf1 to induce the transcription of a subset of genes required for stress adaptation, such as gpd1+, which encodes an enzyme involved in the regulation of the intracellular glycerol concentration, or pyp2+, which encodes the phosphatase Pyp2. Dissociation from phosphorylated Atf1 allows Spc1 to form a complex with the exportin Crm1 in order to be translocated to the cytoplasm. Atf1-dependent transcription of pyp2+ leads to an increase in the cytoplasmic phosphatase activity against Spc1. After export, Spc1 is dephosphorylated in the cytoplasm and resumes association with Wis1.