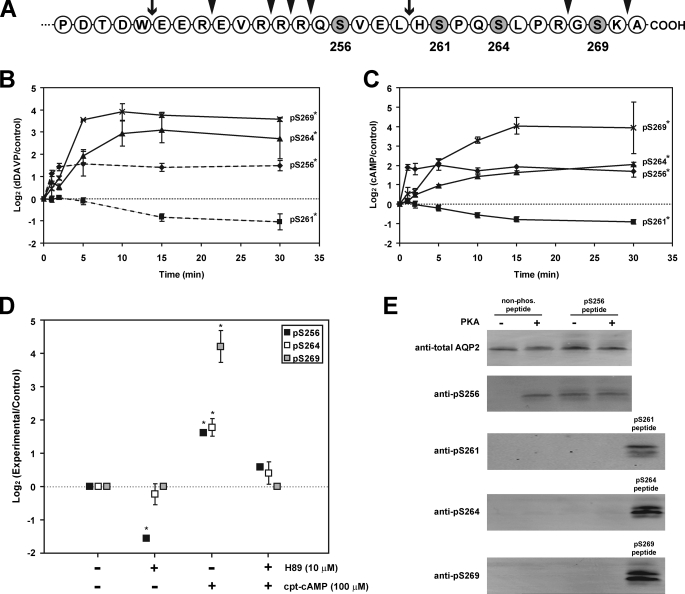
FIGURE 1.
AQP2 polyphosphorylation is altered by vasopressin acting through cyclic AMP and protein kinase A. A, terminal 30 amino acids of COOH-terminal tail of rat AQP2, showing the four serines (shaded) that are phosphorylated. The arrowheads indicate sites of trypsin cleavage; the arrows indicate sites of chymotrypsin cleavage. B, time course of changes in phosphorylation in response to 1 nm dDAVP in rat IMCD tubule suspensions. The results are based on immunoblotting using phospho-specific antibodies recognizing Ser(P)256-, Ser(P)261-, Ser(P)264-, and Ser(P)269-AQP2. Control suspensions for each time point were exposed to vehicle for the same time period. n = 3 for each time point (mean ± S.E.). The data for Ser(P)256- and Ser(P)261-AQP2 were previously reported by Hoffert et al. (12) but are included here for comparison (dashed lines). * indicates area under curve significantly different from zero (p < 0.01) (see supplemental Fig. 2 (top) for examples of the original immunoblots). C, same as A except that 0.1 mm cpt-cAMP was added instead of dDAVP. n = 3 for each time point (mean ± S.E.). * indicates area under curve significantly different from zero (p < 0.01) (see supplemental Fig. 2 (bottom) for examples of the original immunoblots). D, effect of H-89 (10 μm) on COOH-terminal phosphorylation in absence and presence of 0.1 mm cpt-cAMP. n = 3 for each point (mean ± S.E.). * indicates significantly different from zero (p < 0.01). E, in vitro phosphorylation of COOH-terminal AQP2 synthetic peptides by PKA.