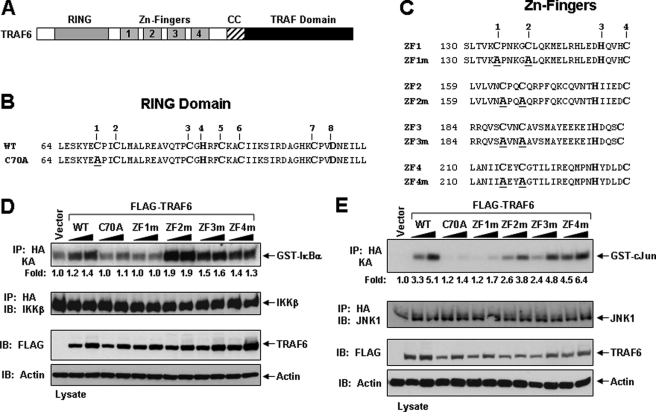
FIGURE 1.
Activation of IKKβ and JNK by TRAF6 and its RING and ZF mutants in HEK293 cells. A, schematic diagram of TRAF6 with the indicated RING, ZF, coiled-coil (CC), and TRAF-C domains. B, the primary amino acid sequence of the RING domain consisting of the conserved Cys and His residues (numbered on top) of mouse TRAF6. The TRAF6-C70A mutation is shown below with the Ala substitution underlined. C, the primary amino acid sequence of the four ZF motifs consisting of the conserved Cys and His residues (numbered on top) of mouse TRAF6. The TRAF6 ZF mutants are shown below with the Ala substitution underlined. D-E, TRAF6-ZF1 is important for IKKβ and JNK activation. HEK293 cells were co-transfected with empty vector (vector) or increasing amounts of the indicated FLAG-tagged TRAF6 constructs together with an HA-tagged IKKβ (D) or JNK (E). Thirty-six hours after transfection, cells were harvested, lysed in Buffer A, and subjected to immunoprecipitation (IP) with an anti-HA antibody followed by an in vitro kinase assay using GST-IκBα (D) or GST-c-Jun (E), respectively, as a substrate. The experiment was performed at least three times with similar results each time. The relative incorporation of 32P into the substrates was quantified by a PhosphorImager and represented as -fold activation as compared with vector. The immunoprecipitates were then immunoblotted (IB) with an IKK (D) or JNK (E) antibody, respectively. Expression of the various TRAF6 proteins in the lysates was detected by immunoblotting with anti-FLAG, and then the membrane was stripped and reprobed with anti-actin.