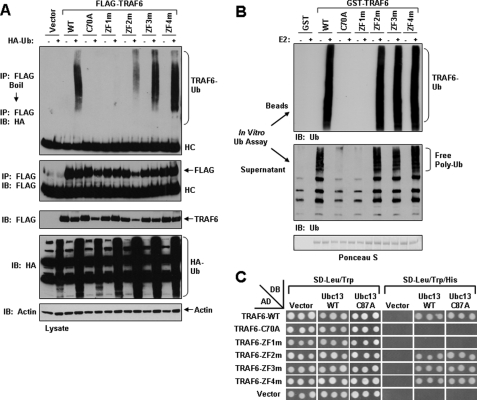
FIGURE 4.
Requirement of the RING and ZF1 domains of TRAF6 for Ub ligase activity. A, overexpressed TRAF6-C70A and TRAF6-ZF1m are not ubiquitinated in HEK293 cells. HEK293 cells were co-transfected with empty vector (vector) or the indicated FLAG-tagged TRAF6 constructs in the absence (-) or presence (+) of HA-tagged Ub. Thirty-six hours after transfection, cells were harvested, and cell lysates were subjected to immunoprecipitation (IP) with an anti-FLAG antibody followed by boiling in 1% SDS and reimmunoprecipitated with anti-FLAG. Finally, the eluted protein was subjected to SDS-PAGE and immunoblotted (IB) with anti-Ub. The membrane was then stripped and reprobed with anti-FLAG. The experiment was performed at least two times with similar results each time. Cell lysates were immunoblotted with the indicated antibodies (bottom). B, bacterially expressed TRAF6-C70A and TRAF6-ZF1m lack ubiquitin ligase activity. The indicated GST fusion proteins bound to glutathione-agarose beads were subjected to an in vitro ubiquitination assay in the absence (-) or presence (+) of Ubc13-Uev1A (E2). After the ubiquitination assay, a portion of the supernatant (middle) was subjected to SDS-PAGE and immunoblotted with anti-Ub. The GST fusion proteins bound to glutathione-agarose beads were washed and then subjected to SDS-PAGE and immunoblotted with anti-Ub (top). The membrane was stained with Ponceau S (bottom). C, TRAF6-C70A and TRAF6-ZF1m do not interact with Ubc13 in yeast. S. cerevisiae strain AH109 was co-transformed with Ubc13 expressed in pGBK-T7 (DB) and the indicated TRAF6 constructs expressed in pGAD-T7 (AD). Following double selection on SD-Trp-Leu, three separate colonies were then picked and spotted onto both SD-Trp-Leu and SD-Trp-Leu-His plates. The plates were incubated at 30 °C until growth appeared on the master plate (SD-Trp-Leu), typically within 2-3 days, and positive interaction was then determined by equivalent growth of colonies on SD-Trp-Leu-His plates.