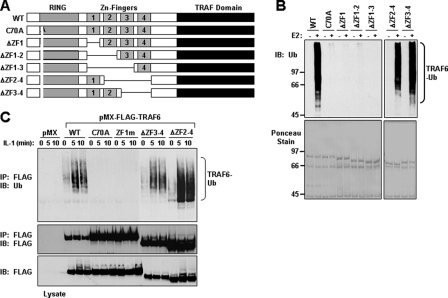
FIGURE 5.
ZF Domains 2-4 are dispensable for TRAF6 autoubiquitination. A, schematic diagram of TRAF6-WT and the internal deletion mutants. B, bacterially expressed TRAF6 mutants lacking ZF1 are not ubiquitinated in vitro. The indicated GST fusion proteins bound to glutathione-agarose beads were subjected to an in vitro ubiquitination assay in the absence (-) or presence (+) of Ubc13-Uev1A (E2). After the ubiquitination assay, the GST fusion proteins bound to glutathione-agarose beads were washed and then subjected to SDS-PAGE and immunoblotted (IB) with anti-Ub (top). The membrane was stained with Ponceau S (bottom). C, TRAF6-C70A and TRAF6-ZF1m are not ubiquitinated following IL-1 stimulation. TRAF6-deficient MEFs stably expressing empty vector (pMX) or the indicated FLAG-tagged TRAF6 constructs were left unstimulated (time 0) or stimulated with IL-1 (10 ng/ml) for the indicated times. Cell lysates were prepared in Buffer A and immunoprecipitated (IP) with anti-FLAG, followed by boiling in 1% SDS, and again immunoprecipitated with anti-FLAG. The samples were then subjected to SDS-PAGE and immunoblotted with anti-Ub (top). The membrane was stripped and reprobed with anti-FLAG (middle). Expression of the TRAF6 proteins in the cell lysates was detected by immunoblotting with anti-FLAG (bottom). The experiment was performed at least two times with similar results each time.