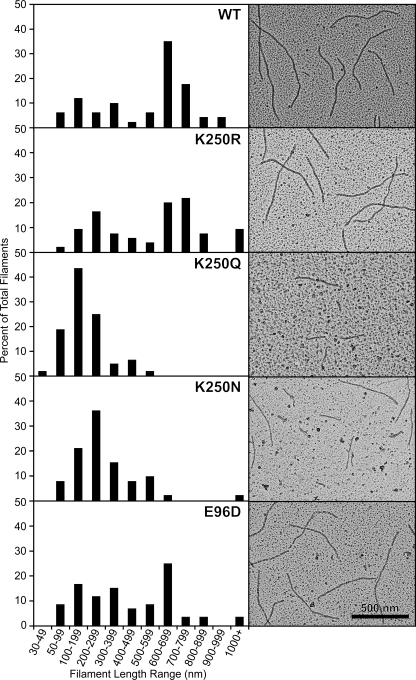
FIGURE 2.
Wild-type and mutant RecA proteins form DNA-dependent nucleoprotein filaments. Electron micrographs show wild-type (WT) and mutant RecA proteins on linear ssDNA. Reactions included 4 μm RecA, 6 μm poly(dT) linear ssDNA, and 3 mm dATP at pH 7.5. The RecA protein was incubated with the DNA at 37 °C for 10 min before the addition of dATP. The reaction was incubated for another 10 min to allow the RecA protein to form a filament on the DNA before ATPγS was added (to 3 mm), and the reaction was incubated for 3 min to stabilize the filaments. Reactions were diluted 10-fold, except the K250Q reaction that was diluted 5-fold, before being adsorbed to the Alcian grid. Nucleoprotein filaments were viewed with the electron microscope. For each RecA protein, a total of at least 52 representative filaments was measured. Filaments shorter than 30 nm were difficult to distinguish from the background and were not included in the reported totals. A histogram of filament length for each protein is shown next to the electron microscopy image. Wild-type, K250R, and E96D proteins formed many filaments greater than 600 nm, implying end-to-end joining of filaments. The K250N and K250Q mutant RecA proteins formed shorter filaments, and K250Q filaments were reduced in concentration. A sample without DNA was examined for each RecA protein to test for DNA-independent filaments; all RecA proteins formed DNA-dependent filaments exclusively.