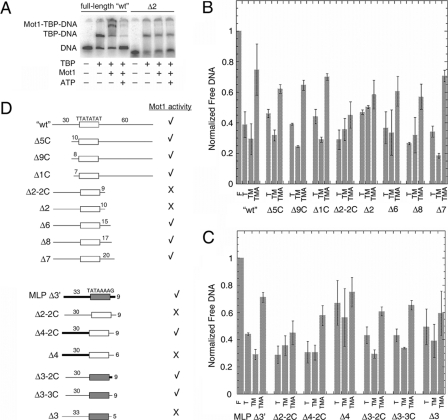
FIGURE 5.
DNA downstream of TATA is required for Mot1 binding and dissociation of TBP from URA1 DNA. A, representative electrophoretic gel mobility shift experiment performed as in Fig. 2A using either radiolabeled WT URA1 DNA or Δ2, a URA1 fragment truncated on the downstream site of the TATA box (see schematic in D). B and C, quantitation of gel mobility shift results for the indicated DNA probes. The bar height represents the relative free DNA in each reaction, ± S.D., determined from at least three independently performed experiments. F, free DNA; T, reaction containing 14 nm TBP; TM, reaction containing 14 nm TBP and 11.6 nm Mot1; TMA, reaction containing 14 nm TBP, 11.6 nm Mot1, and 25 μm ATP. D, schematic of the DNA probes used in the experiments in A-C, and summary of the results. The top set of nine probes was derived from the URA1 promoter. The open rectangle represents the TATA element sequence denoted above it, and the numbers above the flanking DNA segments indicate their lengths in base pairs. The Δ2-2C, Δ2, Δ6, Δ8, and Δ7 probes possess 30 bp of flanking DNA upstream of the TATA sequence and varying lengths of 3′-flanking DNA as indicated. The Δ5, Δ9, and Δ1 probes possess 60 bp of 3′-flanking DNA and varying lengths of 5′-flanking DNA as indicated. The bottom set of seven probes was derived by combining portions of the URA1 and AdMLP promoters, plus appropriate controls. MLP Δ3′ is derived entirely from the AdMLP promoter. In this and other probes in this series, the shaded rectangle represents the AdMLP TATA sequence, TATAAAAG, and the thick black bars indicate AdMLP upstream or downstream sequence. As in the first set of probes, the lengths of flanking DNA, in base pairs, are indicated above the DNA segments. Δ2-2C is shown twice in D simply to make comparison with other constructs easier. Δ4-2C and Δ4 probes possess the indicated lengths of AdMLP flanking DNA upstream of the URA1 TATA box and the indicated lengths of URA1 3′-flanking DNA. Probes Δ3-2C, Δ3-3C, and Δ3 possess the AdMLP TATA sequence but URA1 5′-flanking DNA. The 3′-flanking DNA for Δ3-2C was from the AdMLP promoter, whereas the 3′-flanking DNA for the Δ3-3C and Δ3 probes was derived from the URA1 promoter. A check mark indicates that TBP complexes formed on the indicated probe formed ternary complexes with Mot1 and were dissociated in the presence of Mot1 and ATP. An X indicates that Mot1 binding was poor or undetectable, and little or no catalysis of TBP-DNA dissociation was observed in reactions containing ATP. In no case was ternary complex formation observed without ATP-dependent catalytic activity.