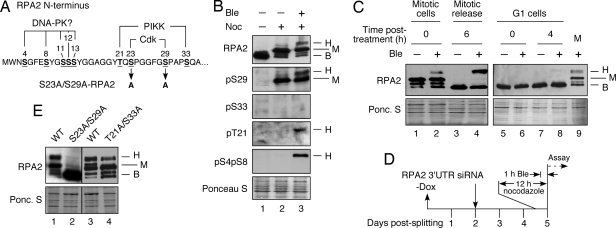
Fig. 1.
Mitotic phosphorylation of RPA2 cyclin-Cdk sites stimulates subsequent stress-dependent modification. (A) Schematic of RPA2 phosphorylation sites. (B) Lysates were isolated from asynchronous nonstressed cells (lane 1) or from nocodazole-arrested cells isolated by mitotic shake-off and then were mock treated (lane 2) or were treated with Ble (2 μg/ml) for 1 h (lane 3). (C) Nocodazole-arrested cells, either treated with Ble (2 μg/ml) for 1 h or not treated, were released into medium lacking both agents and were collected 6 h after release. To examine G1 cells, mitotic cells were released from the nocodazole block and 3 h after release were treated with Ble (2 μg/ml) for 1 h or were mock treated. Cells were collected immediately (0 h) or 4 h after the medium was replaced with medium lacking Ble. (D) Schematic indicating steps involved in cellular RPA2 replacement. −Dox = removal of doxycycline causing the induction of ectopic RPA2 expression. (E) Endogenous RPA2 was silenced in U2-OS cells replacing S23A/S29A-, T21A/S33A-, or WT-RPA2. After silencing (48 h), cells were treated with nocodazole for 12 h. The mitotic cells were collected by shake-off and were treated with Ble (2 μg/ml) for 1 h, followed by collection of cells. For all samples, lysates were prepared from the collected cells and analyzed by Western blotting using the indicated antibodies. The RPA2 species are indicated as follows: B, basal; H, hyperphosphorylated; M, mitotic.