Abstract
Objective
Lithotripters with two shock heads are now available for use in treating patients. However, little information is available by which to judge the safety of treatment with dual pulses. A study was conducted to assess the effect of dual-head lithotripsy on renal function and morphology in a pig model of shock wave (SW) injury.
Methods
A dual-head electrohydraulic lithotripter (Direx Duet) was used to treat the lower renal pole of anesthetized pigs with a clinical dose of SWs (2400 dual SWs; n=10) delivered in synchronous mode (i.e. both heads fired simultaneously). For comparison, pigs were treated with either 2400 SWs (n=12) or 4800 SWs (n=8) with a conventional electrohydraulic lithotripter (Dornier HM3).
Results
Dual SW treatment with the Duet lithotripter caused a decline in glomerular filtration rate (GFR, 4.1 ± 1.9 ml/min) with a trend for effective renal plasma flow (RPF, 31 ± 19 ml/min) to also fall. These changes in renal hemodynamics were comparable to decreases in GFR and RPF in response to treatment with 2400 SWs (4.8 ± 0.8 ml/min and 32 ± 10 ml/min, respectively) or 4800 SWs (5.4 ± 1.0 ml/min and 68 ± 14 ml/min, respectively) with the HM3 lithotripter. Linear association analysis showed that the functional response to dual-pulse SWs was less predictable than with conventional SWs. Morphological quantitation of kidney damage expressed as percentage of functional renal volume (FRV), showed that tissue injury with 2400 paired SWs with the Duet (0.96± 0.39% FRV, n=8) was comparable to injury produced by either 2400 single SWs (1.08±0.38% FRV, n=6), or 4800 single SWs (2.71±1.02% FRV, n=6) with the HM3. However, morphological damage appeared less consistent with the Duet (measurable in only 5 of 8 kidneys) than that observed with the HM3 (measurable in all 12 kidneys). Acoustic output and the timing of dual SWs in synchronous mode increased in variability as the electrodes aged, affecting the amplitude and targeting of focal pressures.
Conclusion
With the caveat that variability in the timing of dual SWs will unpredictably alter the distribution of SW energy within the kidney, this study shows that a clinical dose of dual head SWs delivered in synchronous mode elicits a renal response comparable to, but more variable than, that observed with a clinical dose of SWs from a conventional electrohydraulic lithotripter.
Keywords: lithotripsy, kidney, hemodynamics, morphology, acoustic waves
Introduction
Lithotripters with two treatment heads have recently been approved by the FDA. One such dual head machine, the Direx Duet, can be operated so that the two heads are fired in sequence (alternating mode), or simultaneously (synchronous mode) at rates of up to 120 shock waves (SWs)/min per head. Thus, this lithotripter can deliver 240 individual SWs per minute, twice the number of pulses typically administered with a conventional lithotripter [1]. The manufacturer points to this as a significant advantage, a means to reduce overall treatment time, while working within accepted limits for SW rate [Direxusa.com].
Lithotripsy using dual treatment heads was introduced as a research tool to investigate the role of cavitation in SW action [2]. Studies were conducted with a system in which identical electrohydraulic shock sources were aligned directly facing one another and demonstrated that the timing of paired pulses could be used either to suppress bubble expansion, if the time delay was minimal, or enhance the force of bubble collapse, if the delay was somewhat longer [2]. This led to techniques in which tandem SWs were delivered along the same axis, with the trailing pulse precisely timed to interact with the cavitation initiated by the first pulse [3–5]. This work has been promising, and suggests that with proper timing the trailing SW can be used to suppress the cavitation associated with vascular trauma [6]. For those interested in shortening treatment times in lithotripsy, use of tandem pulses delivered along the same axis poses the limitation that the firing rate of an individual shock source is limited to 120 SW/min by the FDA. So it is that dual head lithotripsy in which both sources can be fired at 120 SW/min is seen as a potential means to achieve improved stone breakage and reduce overall treatment times [7].
Safety is a key issue in shock wave lithotripsy (SWL), and a substantial literature has shown that SWs produce vascular trauma to the kidney [8,9]. Acute damage can progress to a permanent loss of functional renal tissue, and there is evidence that links lithotripsy trauma to significant long-term effects [8–10]. It has also been shown that the severity of renal trauma is dependent on pulse energy [11] and SW number [12], and there is evidence suggesting increased injury with lithotripters that employ a narrow focal zone [13,14]. Thus, the occurrence and severity of tissue trauma is dependent on the acoustic characteristics of the lithotripter.
A lithotripter that delivers SWs using two treatment heads creates conditions of SW exposure that are different from what is produced by a conventional lithotripter. That is, an individual treatment head of a dual head device may be similar to that of a conventional machine, but treatment involves passage of SW energy along two paths through the body, not one, and if the two heads are triggered so that the SWs interact, the acoustic field will be different [2,15]. Accordingly, since virtually all of what is known about SW trauma in lithotripsy comes from experience with conventional lithotripters, one cannot reliably predict what effect dual SWs will have on the kidney.
The present study characterized the renal response to SWs delivered with a Direx Duet dual head electrohydraulic lithotripter. A typical clinical dose of SWs was administered in synchronous mode to an established porcine model of renal trauma and function [11]. The renal response to synchronous dual pulses from the Duet was compared to treatment with the same number of SWs delivered with a Dornier HM3 lithotripter.
Methods and Materials
Adult female swine (45 kg; Hardin Farms, Danville, Indiana) were anesthetized [ketamine (15–20 mg/kg) and xylazine (2 mg/kg) for induction and intubation followed by isofluorane (1–3%) and oxygen (100%) for maintenance] and prepared for renal function measurements as previously described [16]. Catheters were placed into an ear vein for the intravenous infusion of fluids, the infrarenal aorta for blood pressure monitoring and arterial blood sampling, both renal veins for renal venous blood sampling and both ureters for the collection of urine.
Isotonic saline was infused intravenously at 1% of body weight per hour throughout the experiment to maintain adequate hydration and urine flow. Inulin and para-aminohippuric acid (PAH) were infused intravenously at 70 ml/h.
Experimental Protocol for the Direx Duet lithotripter
The pig was in a supine position atop the motorized, adjustable treatment table of the Duet system. The water cushions of the two treatment heads were coupled to the flank of the pig using castor oil as the coupling medium. Baseline cardiovascular and renal function measurements were then begun 30 min later, and consisted of two 25-min clearances. Radiocontrast medium was injected through the left ureteral catheter and the focal zone of the lithotripter was targeted by fluoroscopy on the lower pole calyx of the left kidney. The two study groups with the Duet included animals that were not treated with SWs (Group 1), and animals that received 2400 SWs delivered from each treatment head in synchronous (simultaneous) mode (17 kV, 120 SWs/min) using one pair of electrodes (Group 2). Direx recommended this regimen as representative of clinical treatment in synchronous mode. Lithotripsy was temporarily halted every 500 SWs to confirm targeting. Two 25-min clearances were obtained following a 1-h post-lithotripsy recovery period.
Experimental Protocol for the Dornier HM3 lithotripter
Baseline cardiovascular and renal function measurements were begun 30 min following the completion of all surgery and consisted of two 25-min clearances. The pig was then disconnected from the anesthesia machine and transferred (unconscious) to the lithotripsy suite (a trip of about 5-min) where administration of isofluorane was resumed and the animal positioned supine in the patient gantry of an unmodified Dornier HM3 lithotripter. F2 was targeted on the lower pole calyx of the left kidney using fluoroscopy and aided by administration of radiocontrast material into the collecting system via the left ureteral catheter. The three study groups with the HM3 included animals that were not treated with SWs (Group 3), and those that received either 2400 SWs (Group 4) or 4800 SWs (Group 5) delivered at 24 kV, 120 SWs/min. The latter group was included because both heads of the Duet lithotripter delivered a combined total of 4800 SWs to the kidney. The position of F2 was checked every 500 SWs with the lithotripter in standby mode and the electrode changed at every 1000 SWs. At the end of lithotripsy, the pigs were returned to the surgical suite for two 25-min clearance measurements beginning 1-h post-lithotripsy.
Renal Function Measurements
Plasma and urine samples were analyzed for inulin and PAH, and their renal clearances used as estimates of glomerular filtration rate (GFR) and effective renal plasma flow (RPF), respectively. The renal extraction of PAH (arterial-renal venous PAH difference, EPAH) was measured and provides an estimate of the efficiency of renal tubular PAH transport.
Morphological analysis
The kidneys were perfusion fixed in situ at the end of the experiment [17], and processed for histology and quantitative morphological analysis as previously described [18]. Lesion sizes were quantified for eight of ten kidneys in Group 2 (Duet, 2400 SWs), six of twelve in Group 4 (HM3, 2400 SWs) and six of eight in Group 5 (HM3, 4800 SWs). Lesion size was expressed as a percentage of functional renal volume (FRV) determined by computer-assisted segmentation of digital images collected from serial sections (120 μm) of the entire kidney. Kidneys used to quantifying lesion size could not be used for routine histology because different tissue preparation methods are required for each process. Consequently, kidneys for histological analysis were embedded in paraffin, sectioned at 7 μm and stained with hematoxyline and eosin (H & E).
Acoustics
Characterization of the acoustic output of the Duet lithotripter was performed using a fiber optic hydrophone (FOPH–500, Univ. of Stuttgart, Germany). Measurements were conducted in an acrylic tank having two latex acoustic windows that were coupled to the treatment heads using castor oil. The tank was filled with tap water that was degassed overnight with a pinhole degasser. The degasser was run continuously during measurements, and the oxygen content of the water was measured using a WTW Oxi 330i oxygen meter (Weilheim, Germany). Gas content stabilized at about 2 mg/l (~20% of saturation). For waveform measurements the glass fiber tip was positioned at the geometric focus of the lithotripter, located using the alignment stylus of the Duet. This position was marked by crossed lasers and used for subsequent measurements. Waveforms were collected using the Fast-Frame setup of a Tektronix (TDS 5034) oscilloscope and post-processed using programs written in LabVIEW (National Instruments, Austin, TX). A photodiode was used to assess the time delay between the firing of the electrodes. The photodiode was positioned closer to one of the treatment heads, so as to distinguish the signal coming from that head.
Statistical Analysis
All measurements were considered numerical variables and summarized as mean ± standard error of the mean. Mixed effect models were used to assess the association of measurements to factors such as lithotripter (Duet vs. HM3), treatment (control vs. SWs), time (before vs. 1 h post SWL), kidney (shocked vs. non-shocked) and their interactions. Multiple comparisons of means were made by Tukey’s adjustment tests. P values less than 5% were considered statistically significant. Pearson correlation coefficients were used to assess the linear relationship of measurements of the SWL-treated kidney. All statistical analyses were performed using SAS 9.1 software (SAS, Gary, NC).
Results
Functional measurements: Cardiovascular and Renal hemodynamics
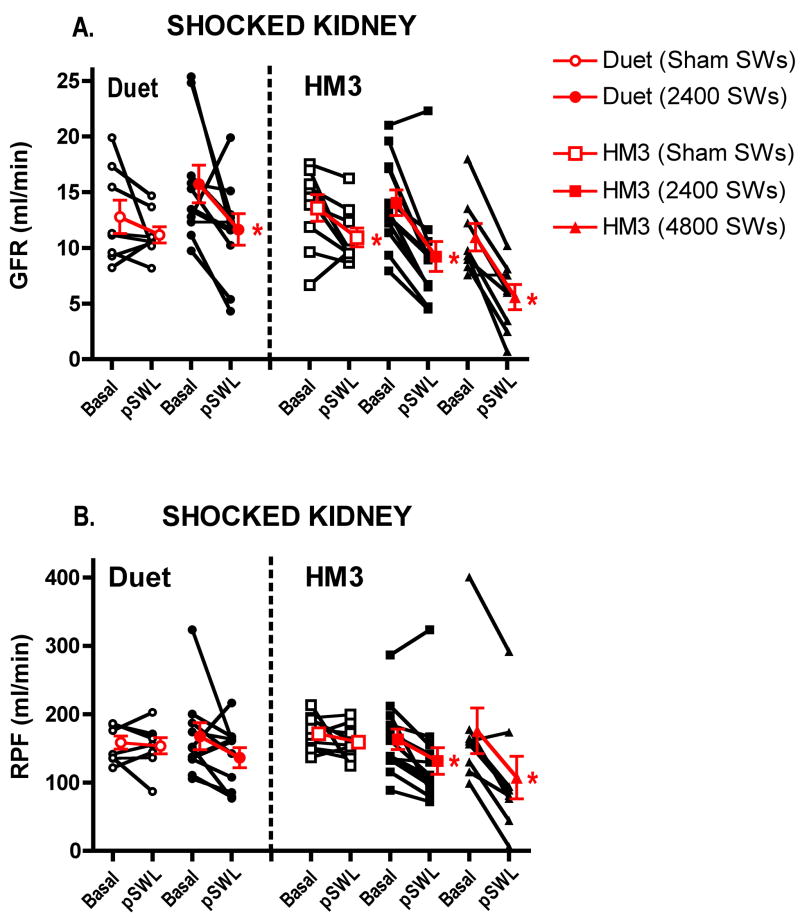
The two Duet lithotripter groups had similar values for blood pressure and renal function, as did the three HM3 lithotripter groups (Table 1). Mean arterial pressure (MAP) and EPAH remained unchanged from baseline values following treatment in all five groups, except in sham Duet lithotripter-treated animals (Group 1) that showed a fall in MAP of 9 ± 2 mm Hg (P<0.05) over the course of the experiment. Renal hemodynamic responses of the SWL- and sham-treated kidney are shown in figure 1
Table 1.
Basal values of cardiovascular and renal function
| MAP (mm Hg) | GFR (ml/min) | RPF (ml/min) | EPAH (%) | |
|---|---|---|---|---|
| Duet lithotripter | ||||
| Group 1 (sham, N = 8) | 80 ± 3 | 12.8 ± 1.5 | 159 ± 10 | 86 ± 3 |
| Group 2 (2400 SWs, N = 10) | 82 ± 2 | 15.7 ± 1.7 | 168 ± 20 | 87 ± 2 |
| HM3 lithotripter | ||||
| Group 3 (sham, N = 9) | 69 ± 3 | 13.6 ± 1.2 | 172 ± 8 | 83 ± 2 |
| Group 4 (2400 SWs, N = 12) | 75 ± 3 | 14.1 ± 1.1 | 163 ± 15 | 87 ± 2 |
| Group 5 (4800 SWs, N = 8) | 72 ± 2 | 11.0 ± 1.2 | 176 ± 34 | 83 ± 2 |
MAP = mean arterial blood pressure, GFR = glomerular filtration rate, RPF = effective renal plasma flow, EPAH = renal extraction of PAH, N = number of animals. Values are mean ± standard error.
Figure 1.
Renal filtration and perfusion responses in the SWL-treated kidney. Individual responses are shown with black lines, and the mean and Standard Error of the Mean (SEM) value of the group response are shown in red. GFR = glomerular filtration rate, RPF = effective renal plasma flow, Basal = baseline value, pSWL = 1 h post-treatment (sham or SWL) value, * = P<0.05 from baseline value.
Direx Duet lithotripter
GFR and RPF remained constant in time-control animals (Group 1). Dual-pulse SW application to Group 2 animals resulted in a decline in GFR of 4.1 ± 1.9 ml/min (P=0.058). RPF declined in 8 of 10 pigs, but as a group did not reach a level of statistical significance (31 ± 19 ml/min, P=0.129).
Contralateral renal filtration and perfusion was unchanged in Group 1 pigs, whereas Group 2 pigs showed a reduction in GFR of 3.2 ± 1.2 ml/min (P<0.05) and a fall in RPF in 7 of 10 pigs that, as a group, did not reach statistical significance (25 ± 19 ml/min, P=0.217) (not shown).
Dornier HM3 lithotripter
Sham-treated animals (Group 3) showed a time-related fall in GFR of 2.6 ± 1.1 ml/min (P<0.05) whilst RPF remained unaltered. Treating pig kidneys with 2400 SWs (Group 4) resulted in a fall in GFR and RPF of 4.8 ± 0.8 ml/min and 32 ± 10 ml/min, respectively (P<0.01 each). Application of 4800 SWs to the kidneys of Group 5 reduced GFR and RPF by 5.4 ± 1.0 ml/min and 68 ± 14 ml/min, respectively (P<0.01 each). A fall in GFR of 21 to 34% was still detectable in the HM3 lithotripter-treated animals after factoring the time-related fall in GFR observed in the sham HM3 lithotripter-treated animals (not shown).
The contralateral kidneys of Group 3 pigs had no significant change in GFR or RPF, whereas Group 4 showed a fall in GFR and RPF of 2.9 ± 1.0 ml/min (P<0.05) and 17 ± 8 ml/min (P=0.053), respectively, and Group 5 had declines in GFR and RPF of 3.3 ± 1.1 ml/min and 31 ± 12 ml/min, respectively (P<0.05 each) (not shown).
Direx Duet vs. Dornier HM3 lithotripter
Overall, the magnitude of the GFR and RPF response in the shocked kidney was similar in the Duet lithotripter-treated and HM3 lithotripter-treated animals (see above). However, HM3 lithotripter-treated groups had higher (>60%) and significant correlation coefficients for GFR and RPF, whereas lower (<50%) and insignificant correlation coefficients were observed in the Duetlithotripter-treated group (Table 2). A higher correlation coefficient indicated that functional changes in GFR and/or RPF were more predictable after SWL.
Table 2.
Pearson correlation coefficients (r-value) were used to assess the degree of closeness of the linear relationship between values before and after SWL. A perfect correlation has a r-value of 1
| Measurement | Lithotripter | N | SW number | r-value | P-value |
|---|---|---|---|---|---|
| GFR | Duet | 10 | 2400 | 0.28 | 0.428 |
| HM3 | 12 | 2400 | 0.78 | 0.003 | |
| HM3 | 8 | 4800 | 0.67 | 0.071 | |
| RPF | Duet | 10 | 2400 | 0.45 | 0.192 |
| HM3 | 12 | 2400 | 0.87 | 0.001 | |
| HM3 | 8 | 4800 | 0.91 | 0.002 |
Morphological measurements
Gross Morphology
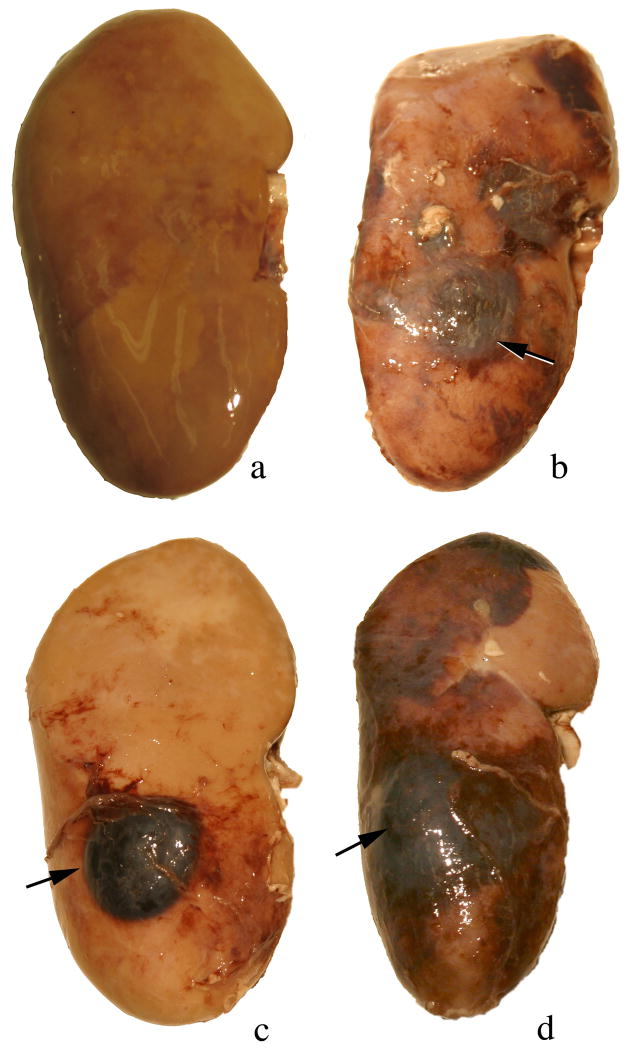
At the time of autopsy, all kidneys were examined for evidence of subcapsular hemorrhage. No sites of hemorrhage were seen on any of the sham-treated (control) kidneys (Figure 2a). Two of the ten Duet-treated kidneys showed sites of subcapsular bleeding: one kidney showed several small sites of subcapsular bleeding (not shown) and a second kidney showed a well defined subcapsular hematoma in the targeted pole (Figure 2b), while nine of the twelve HM3 2400 SW-treated kidneys (Figure 2c) and five of the eight HM3 4800 SW treated kidneys (Figure 2d) showed a subcapsular hematoma in the targeted pole that was always large in size
Figure 2.
Gross morphology of kidneys at the time of autopsy. No sites of subcapsular hemorrhage were seen on any of the sham-treated kidneys (panel a). Two of the ten Duet treated kidneys showed sites of subcapsular bleeding with one kidney having a well defined subcapsular hematoma in the targeted pole (arrow, panel b), while nine of the twelve HM3 2400 SW treated kidneys (arrow, panel c) and five of the seven HM3 4800 SW treated kidneys (arrow, panel d) showed well defined subcapsular hematomas that were always large in size. Magnification, ×1.2 (a–d).
Histopathology
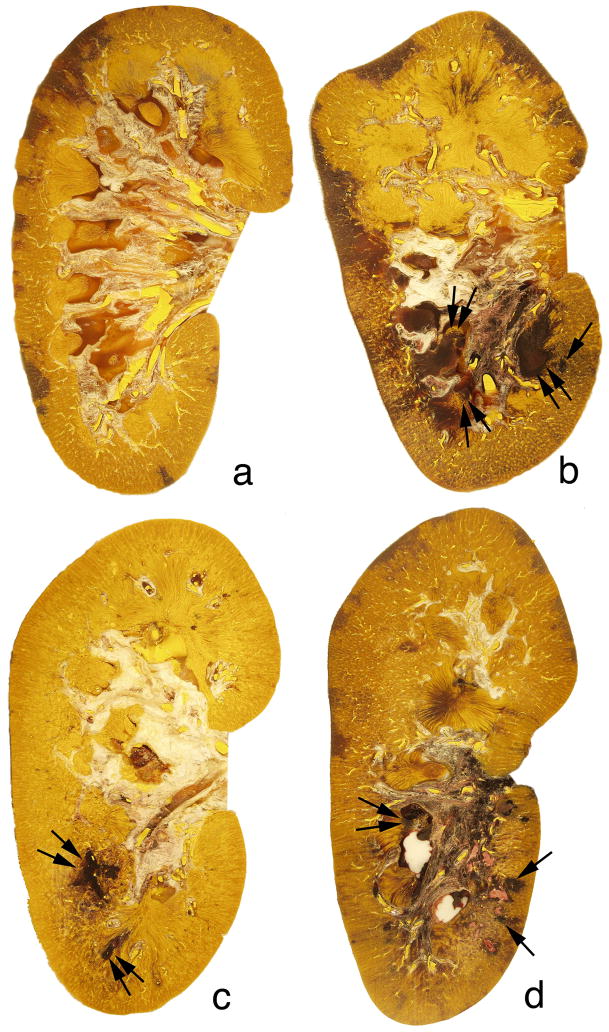
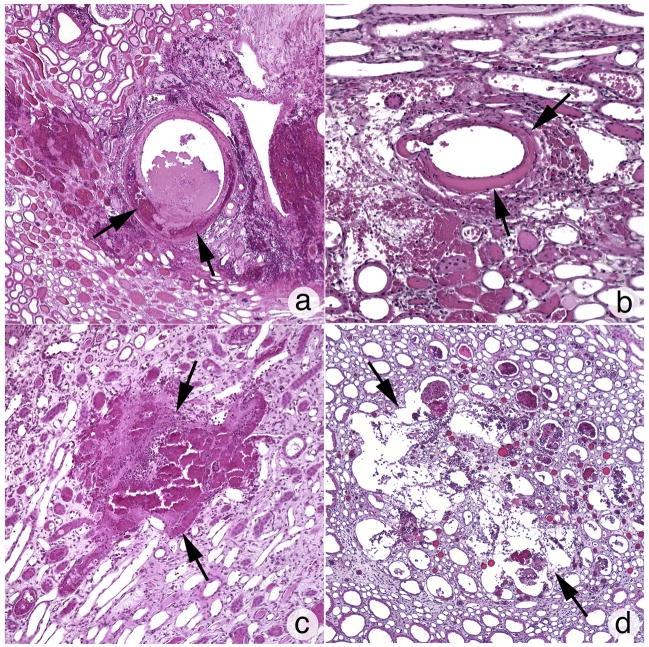
The degree of intraparenchymal hemorrhage induced by the Duet lithotripter was variable and included one pig with no detectable lesion, two pigs with a lesion below the sensitivity of accurate measurement and assigned a threshold value of 0.1% FRV, and one pig with the largest lesion size of 3.16% FRV. The mean and standard error for the eight Duet-treated kidneys was 0.96 ± 0.39% FRV (range=0 – 3.16% FRV). All kidneys treated with the HM3 lithotripter had a measurable lesion (2400 SWs: 1.08 ± 0.38% FRV, range=0.28 –2.50% FRV, n=6; 4800 SWs: 2.71 ± 1.02% FRV, range=1.55 – 7.02% FRV, n=6). Figure 3a shows a digitized and colorized cross-section from the Duet lithotripter-treated kidney that showed no detectable lesion, while figure 3b shows a cross-section from the kidney with the largest lesion induced by the Duet lithotripter (i.e. 3.16% FRV). Sites of intraparenchymal hemorrhage were seen in the medulla and cortex, a pattern of injury that was similar to kidneys treated with the HM3 lithotripter at 2400 SWs (Figure 3c) or 4800 SWs (Figure 3d). Cortical damage with the Duet was primarily localized to the walls of veins and arteries within the area of the kidney targeted by the focal zone. Damage varied from dissection of the tunica media, including injury to smooth muscle cells (Figure 4a and 4b), to rupture of the vessel wall permitting release of blood into the interstitial space. Histopathologic examination of damaged papilla from the Duet lithotripter- and HM3 lithotripter-treated kidneys showed cellular disruption of vasa recta resulting in intraparenchymal hemorrhage and injury to nearby tubules, with cellular necrosis and tearing of the tubular basement membrane (Figure 4c and 4d).
Figure 3.
Digitized and colorized cross-sections of Duet-treated and HM3-treated kidneys. The degree of intraparenchymal hemorrhage induced by the Duet lithotripter varied from no detectable lesion in one animal (panel a) to a lesion size of 3.16% of FRV (panel b). The sites of intraparenchymal hemorrhage were noted in the papilla (double arrows) and adjacent cortical tissue (arrow) within the focal zone. All kidneys that received 2400 SWs (panel c) or 4800 SWs (panel d) from the HM3 lithotripter had lesions similar to that seen in the Duet-treated kidneys. Sites of intraparenchymal hemorrhage were seen in the medulla (double arrow) and cortex (arrows). Magnification, ×1.2 (a–d).
Figure 4.
Histopathologic examination of sites of cortical and medullary damage induced by the Duet and HM3 lithotripters. Cortical damage was primarily localized to the walls of veins and arteries within the area targeted by the focal zone, and varied from dissection of the tunica media with blood cells and damaged smooth muscle cells (arrows, panels a and b) to rupturing of the vessel wall permitting the release of blood into the interstitial space. Histopathologic examination of damaged papilla from both the Duet- and HM3-treated kidneys show cellular distruption of vasa recta resulting in intraparenchymal hemorrhage (arrows panel c) and injury to nearby tubules that included cellular necrosis and tearing of the tubular basement membrane (arrows, panel d). The pattern of cortical and medullary injury was similar between the two lithotripters. Magnification, x1,200 (a); ×1,000 (b–d).
Acoustic measurements
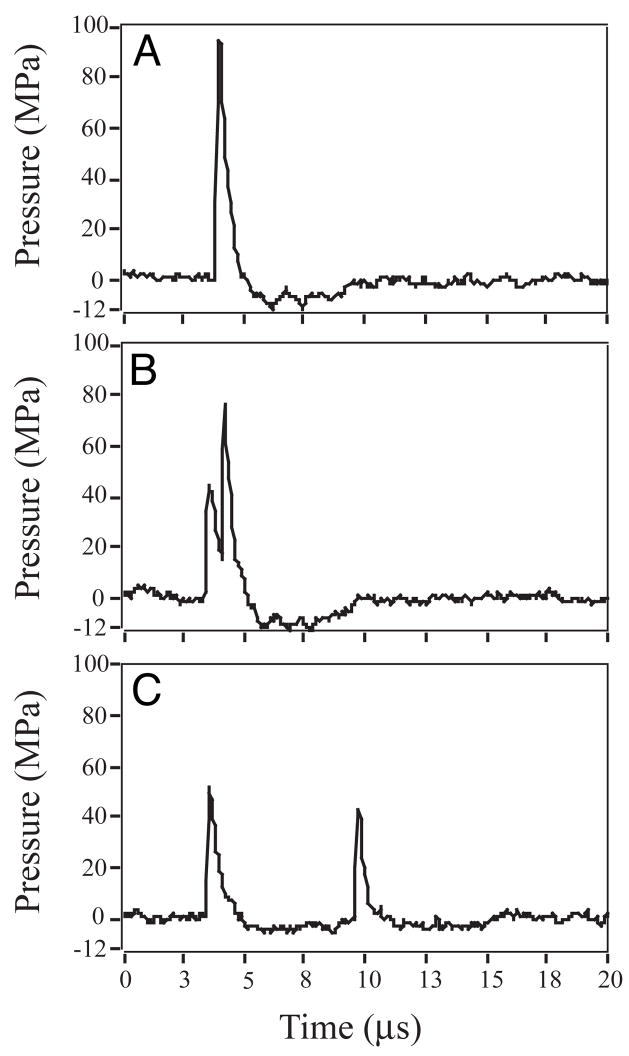
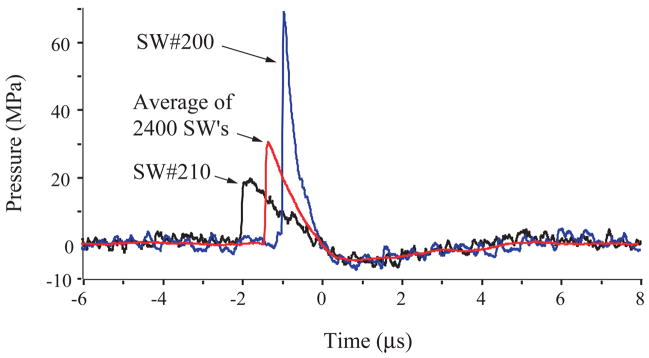
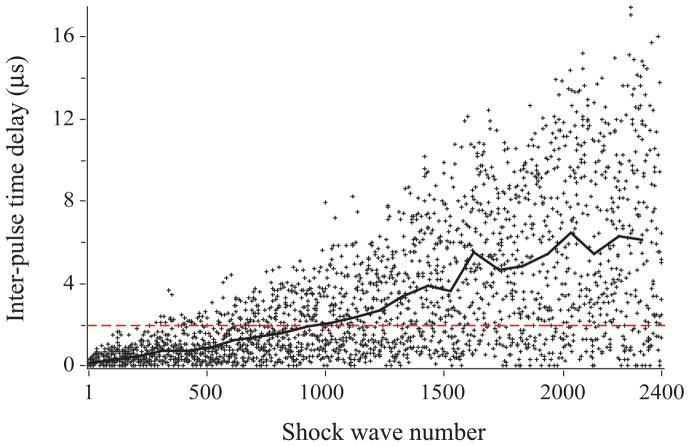
The Duet lithotripter fired shock pulses from both treatment heads in relatively close synchrony, but with an increase in inter-pulse delay as the electrodes aged. When firing was precisely synchronous, the resulting waveform had a peak positive pressure of 40 to 120 MPa, 1 to 2 μs duration, and a negative pressure trough of ~5 MPa (Fig. 5). The waveform from an individual treatment head had about half this amplitude (Fig. 6). The acoustic output of the individual heads was variable, and the amplitude of the peak positive pressure of consecutive pulses could differ by a factor of two. When timing of the dual pulses was close, but not perfectly synchronous, the waveform had two peaks (Fig. 5). Typically, the second peak was higher than the first, likely due to summation of the acoustic pressures. With an increasing number of shots fired on a set of electrodes, the time delay between the dual pulses increased. A delay of ~3μs produced two separate waveforms, each typical of the SWs from a single source. The decay in pulse synchrony over the lifetime of the electrodes was documented by recording the inter-pulse delay detected using a photodiode. Figure 7 shows that as the number of shots increased, the inter-pulse delay increased. Synchrony was near perfect for the first 500 dual pulses, and through 1000 pulse pairs ~80% were separated by ~2μs or less—excellent synchrony. Over the 2400 SW manufacturer-recommended lifetime of the electrodes, 49% of the pulses had a delay of 2μs or less, and for 14% of dual pulses the delay was 7μs or greater. The last 100 paired pulses showed a mean inter-pulse delay of ~6μs, and about 80% of these dual pulses were completely separate (>2 μs delay).
Figure 5.
The effect of inter-pulse time delay on waveforms from the Duet lithotripter fired at 17kV. (A) When the two electrodes fired in precise synchrony (with no inter-pulse delay), acoustic pressures at the focal point of the lithotripter were about twice that produced by a single electrode. (B) A slight delay in timing (<1μs) resulted in two peaks, the second of which was typically higher in amplitude. (C) A longer delay (6μs) between the firing of the electrodes separated the peaks, which were correspondingly lower in amplitude, and similar to SWs fired from a single source.
Figure 6.
Acoustic output for a single shock source of the Duet lithotripter fired at 17 kV, 1Hz. Shown here are waveforms for two shock pulses selected to demonstrate the variability in output from a single shock source (bottom head) in the Duet. SW 210 had low amplitude (~20 MPa peak P+) and relatively long pulse duration (~2μs), while SW 200 was high amplitude (~70 MPa peak P+) and shorter duration (~1μs). Also shown is the trace generated from the average of values over the recommended lifetime (2400 SWs) of the electrode (30 MPa P+, duration 1.5μs, ~5MPa P−).
Figure 7.
Photodiode measures of inter-pulse delay for a set of Duet electrodes fired in synchronous mode. Early in the life of the electrodes, firing was nearly simultaneous and the majority of paired pulses were separated by a delay of ≤2 μs (dashed line). As the electrodes aged, the inter-pulse delay increased, such that during the last 1400 paired pulses only ~27% were simultaneous (≤2 μs). Solid line tracks mean values for inter-pulse delay at 100 shot intervals.
Discussion
In the present study, we found that pig kidneys treated with a clinical dose of synchronous SWs from the Duet lithotripter exhibited a relatively small post-treatment fall in glomerular filtration rate and renal perfusion. Overall, these responses were similar to those observed in kidneys exposed to 2400 SWs with the HM3, and slightly less than that for the 4800 SW HM3 treated group. All animals treated with the Duet or the HM3 lithotripters showed some degree of fall in renal function in the non-targeted kidneys. This was not altogether unexpected, as SW treatment of one kidney has been shown to impair bilateral renal function in animals [16,19] and humans [20] alike. The functional response to SW treatment with the Duet lithotripter, although similar in magnitude to that observed with the HM3, was less predictable (i.e. more variable). Pearson correlation coefficients demonstrated a strong linear relationship between the renal hemodynamic responses for both doses of SWs with the HM3 lithotripter, but not with the Duet lithotripter.
Histological analysis and morphological quantitation of the hemorrhagic lesion in kidneys revealed that tissue injury upon treatment with 2400 paired SWs with the Duet was comparable to the injury produced by 2400 SWs with the HM3. Like the functional response to treatment, morphological damage with the Duet appeared less consistent than that observed with the HM3. Whereas, all HM3 treated kidneys showed visible damage and quantifiable lesions, 3 of 8 kidneys in the Duet-treated group had lesions below the sensitivity of measurement, and in one of these kidneys no lesion was visible.
Thus, the data suggest that by measures of both morphological tissue damage and renal functional response, the injury to pigs treated with synchronous dual SWs was similar to, although more variable than, that of animals treated with the same number of SWs from a conventional lithotripter. Although this may be viewed as an encouraging result, we urge caution in interpreting what this means. Whereas, the study design compared the same number of SWs with both lithotripters, one cannot conclude that treatment with these two machines was equivalent. All lithotripters are similar in the sense that they produce a signature waveform but in some respects this is where the similarity ends. No two lithotripters combine precisely the same shock source and focusing mechanism, and as such their acoustic output—and in particular the distribution of energy within the focal volume—will be different. Two different lithotripters will generate different acoustic fields even if they are operated at the same kilovoltage. Also, the dimensions and energy flux densities that characterize the focal volumes of different machines will be different even when they are operated to deliver the same acoustic pressure. Thus, the treatment groups in this study did not receive equivalent doses of SW energy. Still, this comparison is relevant in the sense that the SW doses delivered with the Duet and the HM3 were typical of the doses used to treat patients with these respective lithotripters.
The rationale behind the use of simultaneous SWs from two separate treatment heads builds on the idea that two relatively low energy pulses can be used to deliver twice the energy to a common focal volume. That is, if the timing between the pulses is synchronous their focal volumes will intersect, creating a focal zone of coincidence with features different from that of a single shock source fired separately. One would predict that acoustic pressures within this zone would be additive of the two separate pulses, and this is what we measured. One would also predict that the dimensions of the zone of coincidence and the focal zone of a single pulse would differ, but it is difficult to say by how much. Direct mapping of the dual pulse field in the Duet is made difficult by the great shot to shot variability of the pulses. Preliminary results of two-dimensional modeling using values for a single pulse as input data suggests that there will be both constructive and destructive interference as the pulses interact [unpublished, YAP]. If there is a delay between the pulses—if they are not simultaneous—there will be spatial drift in the zone of coincidence, and if the delay is 7μs or greater, the two SWs will miss one another altogether.
We observed degradation in the synchrony of dual pulses with the Duet. Early in the life of electrode pairs the individual SWs arrived at the hydrophone with little to no delay separating them. As the electrodes aged, however, the average delay between the paired pulses became longer. About 95% of the first 500 paired pulses had a delay of ~2 μs or less, and of the first 1000 pulses ~80% were within this short delay. Of the remaining 1400 paired pulses of electrode lifetime only ~27% were simultaneous (<2μs delay), and ~25% had a delay greater than 7 μs. Preliminary in vitro studies with the Duet suggest that such a change in inter-pulse delay can have a dramatic effect on the efficiency of stone breakage. For example, we have observed that stone breakage is more efficient during the first thousand dual pulses than during the second thousand, and when pulse timing is purposely manipulated to give a 10μs delay, breakage with delayed dual pulses is significantly less efficient than with pulses delivered by one shock head alone [21].
A measured delay between dual pulses means that they will not arrive at the geometric focus of the lithotripter at precisely the same time. This does not mean, however, that they will not intersect elsewhere. A lithotripter SW advances at ~1.5 mm/μs. Since the shock heads of the Duet are oriented at an angle of 72°, a delay of 6rs will shift the point of coincidence by ~8.6 mm—that is, move the axis of the focal zone nearly a centimeter. Numerous SW pairs were separated by such a delay, and the time delay for some was as much as ~16 μs, for which the displacement of the focal axis would be substantial (~1.5 cm). Considering the variability in pulse delay that we observed over the lifetime of the electrodes, it is clear that the focal zone could not have stayed within just one region of the kidney. It seems reasonable to suggest that this predicted shift in targeting could have been a factor in the variability we observed in functional response and tissue damage in the Duet treated animals.
Movement of the focal zone with the Duet might also have affected the severity of morphological damage to the kidney. In studies with the HM3, we have observed that SW damage is relatively consistent and that the lesion is focal [8,22]. There can be visible damage outside the targeted region, but quantifiable injury is typically limited to one area of tissue. Our group has shown that injury with the HM3 jumps from ~0.2% FRV at 1000 SWs, to ~6.1% FRV at 2000 SWs [16,23]. Thus, the vast majority of the measurable damage occurs after 1000 SWs. It seems reasonable to speculate that a threshold exists for significant hemorrhagic injury with the HM3, and that it exceeds 1000 SWs. It also seems reasonable that if targeting were to be shifted before the threshold was reached, tissue damage would be reduced. This could be the case during treatment with the Duet in synchronous mode. That is, repetitive sets of simultaneous paired SWs would be expected to hit the same area of the kidney. As synchrony degrades and targeting becomes less consistent, sequential SW pairs no longer impact the same region of tissue, and it takes more SWs to reach the threshold for causing significant hemorrhagic injury. This suggests the possibility that if a larger percentage of dual SWs had been truly simultaneous, damage to the kidney would have been more severe.
Acoustic coupling is another factor that could have contributed to the variability in biological response we observed with the Duet treated animals. Recent in vitro studies have shown that the coupling interface of dry-head lithotripters is prone to catching air pockets that can have a significant effect on the transmission of SW energy [24]. Air pockets occur even under controlled in vitro conditions, and the quality of the interface can be remarkably different from one coupling to the next [25]. Clearly the potential for ineffective coupling is a problem common to all dry-head machines, but it seems reasonable that there may be additional difficulty in achieving and maintaining adequate coupling when using lithotripters with two treatment heads.
Another dual head lithotripter has been used in clinical practice. This device (TwinHeads, FMD Corp.) employs two electrohydraulic shock sources, one in fixed position under the treatment table, the other movable. Although several reports have been published describing in vitro stone breakage, assessment of renal injury and initial clinical experience with this lithotripter operated in synchronous (simultaneous) mode [26–29], it has recently been disclosed by the manufacturer that dual pulses with the TwinHeads are actually separated by a lengthy delay (~23 milliseconds) [U.S. Patent 6780161]. The influence of pulse timing on stone breakage and tissue injury in dual head lithotripsy has yet to be adequately defined, and it may be that a certain delay time is advantageous—but this needs to be determined with rigorous systematic testing.
In conclusion, this study shows that a clinical dose of paired SWs delivered to the pig kidney with a dual head electrohydraulic lithotripter (Direx Duet) operated in synchronous mode elicits a renal response comparable to, but more variable than, that observed in the same animal model treated with a clinical dose of SWs from a conventional electrohydraulic lithotripter (unmodified Dornier HM3). This finding is tempered, however, by observation that the timing of dual pulses degrades as the electrodes age, affecting targeting of the focal volume and altering the distribution of SW energy—conditions that will reduce the energy flux density of SW exposure to the kidney.
As synchronous pulse delivery—simultaneous pulses from both heads— is just one option with dual treatment heads, there is need to assess stone breakage and the renal response to SW delivery in alternating mode. Also, since previous experiments with dual shock heads, and complementary studies of tandem SWs delivered along one treatment path suggest that pulse timing can be used to enhance stone breakage and minimize tissue injury, it seems reasonable that continued studies to assess the potential advantages of pulse timing in dual head lithotripsy are warranted.
Acknowledgments
The authors are grateful for the expert services of Philip M. Blomgren, Kelli R. Wind and Cynthia D. Johnson. We thank Dr. Michael R. Bailey for reviewing this manuscript and for his helpful comments. National Institute of Health Grants DK43881 and DK55674 supported this study.
References
- 1.Lingeman JE. Lithotripsy systems. In: Smith AD, Badlani GH, Bagley DH, Clayman RV, Docimo SG, Jordan GH, Kavoussi LR, Lee BR, Lingeman JE, Preminger GM, Segura JW, editors. Smith’s Textbook on Endourology. Hamilton, Ontario, Canada: BC Decker, Inc; 2007. pp. 333–42. [Google Scholar]
- 2.Bailey MR. Control of acoustic cavitation with application to lithotripsy. University of Texas; Austin: 1997. [Ph.D. dissertation] [Google Scholar]
- 3.Zhong P, Cocks FH, Cioanta I, Preminger GM. Controlled, forced collapse of cavitation bubbles for improved stone fragmentation during shock wave lithotripsy. J Urol. 1997;158:2323–8. doi: 10.1016/s0022-5347(01)68243-0. [DOI] [PubMed] [Google Scholar]
- 4.Huber P, Debus J, Jöchle K, et al. Control of cavitation activity by different shockwave pulsing regimes. Phys Med Biol. 1999;44:1427–37. doi: 10.1088/0031-9155/44/6/301. [DOI] [PubMed] [Google Scholar]
- 5.Loske AM, Fernandez F, Zendejas H, Paredes M, Castano-Tostado E. Dual pulse shock wave lithotripsy: in vitro and in vivo study. J Urol. 2005;174:2388–92. doi: 10.1097/01.ju.0000180416.03512.24. [DOI] [PubMed] [Google Scholar]
- 6.Zhong P, Zhou Y. Suppresion of large intraluminal bubble expansion in shock wave lithotripsy without compromising stone comminution: methodology and in vitro experiments. J Acoust Soc Am. 2001;110:3283–91. doi: 10.1121/1.1416906. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein A, Sofer M, Matzkin H. Efficacy of the Duet lithotripter using two energy sources for stone fragmentation by shockwaves: an in vitro study. J Endourology. 2004;18:942–5. doi: 10.1089/end.2004.18.942. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998;78:1–8. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 9.Lingeman J, Delius M, Evan A, et al. Bioeffects and physical mechanisms of SW effects inSWL. In: Segura J, Conort P, Khoury S, Pak C, Preminger GM, Tolley D, editors. Stone Disease: First International Consultation on Stone Disease. Health Publications; Paris: 2003. pp. 251–86. [Google Scholar]
- 10.Krambeck AE, Gettman MT, Rohlinger AL, Lohse GM, Patterson DE, Segura JW. Diabetes Mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742–7. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 11.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–8. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 12.Willis LR, Evan AP, Connors BA, et al. Shockwave lithotripsy: dose-related effects on renal structure, hemodynamics, and tubular function. J Endourology. 2005;19:90–101. doi: 10.1089/end.2005.19.90. [DOI] [PubMed] [Google Scholar]
- 13.Kohrmann KU, Rassweiler JJ, Manning M. The clinical introduction of a third generation lithotripter Modulith SL 20. J Urol. 1995;153:1379–83. [PubMed] [Google Scholar]
- 14.Dhar NB, Thornton J, Karafa MT, Streem SB. A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urol. 2004;172:2271–4. doi: 10.1097/01.ju.0000143459.03836.2d. [DOI] [PubMed] [Google Scholar]
- 15.Sokolov DL, Bailey MR, Crum LA. Use of a dual-pulse lithotripter to generate a localized and intensified cavitation field. J Acoust Soc Am. 2001;110:1685–95. doi: 10.1121/1.1394221. [DOI] [PubMed] [Google Scholar]
- 16.Willis LR, Evan AP, Connors BA, et al. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Neph. 1999;10:1753–62. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 17.Evan AP, Hay DA, Dail WG. SEM of the proximal tubule of the adult rabbit kidney. Anat Rec. 1978;191:397–413. doi: 10.1002/ar.1091910402. [DOI] [PubMed] [Google Scholar]
- 18.Blomgren PM, Connors BA, Lingeman JE, Willis LR, Evan AP. Quantitation of shock wave lithotripsy-induced lesion in small and large pig kidneys. Anat Rec. 1997;249:341–8. doi: 10.1002/(SICI)1097-0185(199711)249:3<341::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Connors BA, Evan AP, Willis LR, et al. Renal nerves mediate changes in contralateral renal blood flow after extracorporeal shockwave lithotripsy. Nephron Physiol. 2003;95:67–75. doi: 10.1159/000074843. [DOI] [PubMed] [Google Scholar]
- 20.Nazaroglu H, Akay AF, Bükte Y, Sahin H, Akkus Z, Bilici A. Effects of extracorporeal shock-wave lithotripsy on intrarenal resistive index. Scand J Urol Nephrol. 2003;37:408–12. doi: 10.1080/00365590310006354. [DOI] [PubMed] [Google Scholar]
- 21.McAteer JA, Pishchalnikov YA, Pishchalnikova IV, et al. Importance of pulse synchrony to stone comminution in dual-pulse lithotripsy: independent characterization of the Direx Duet dual-pulse lithotripter. J Urol. 2004;171:443. [abstract] [Google Scholar]
- 22.Shao Y, Connors BA, Evan AP, Willis LR, Lifshitz DA, Lingeman JE. Morphological changes induced in the pig kidney by extracorporeal shock wave lithotripsy: nephron injury. Anat Rec. 2003;275A:979–89. doi: 10.1002/ar.a.10115. [DOI] [PubMed] [Google Scholar]
- 23.Connors BA, Evan AP, Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourology. doi: 10.1089/end.2006.20.607. (In Press) [DOI] [PubMed] [Google Scholar]
- 24.Pishchalnikov YA, Beard S, Pishchalnikova IV, Williams JC, Jr, McAteer JA. Bubbles trapped at the coupling surface of the treatment head significantly reduce acoustic energy delivered in shock wave lithotripsy. 5th Int’l Symp Therapeutic Ultrasound. AIP Conf Proc. 2006;829:643–7. [Google Scholar]
- 25.Pishchalnikov YA, Neucks JS, VonDerHaar RJ, Pishchalnikova IV, Williams JC, McAteer JA. Air pockets trapped during routine coupling in dry-head lithotripsy can significantly reduce the delivery of shock wave energy. J Urol. doi: 10.1016/j.juro.2006.07.149. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheir KZ, El-Sheikh AM, Ghoneim MA. Synchronous twin-pulse technique to improve efficacy of SWL: Preliminary results of an experimental study. J Endourology. 2001;15:965–75. doi: 10.1089/089277901317203029. [DOI] [PubMed] [Google Scholar]
- 27.Sheir KZ, Zabihi N, Lee D, et al. Evaluation of synchronous twin pulse technique for shock wave lithotripsy: Determination of optimal parameters for in vitro stone fragmentation. J Urol. 2003;170:2190–4. doi: 10.1097/01.ju.0000094188.69698.f8. [DOI] [PubMed] [Google Scholar]
- 28.Sheir KZ, Lee D, Humphrey PA, Morrisey K, Sundaram CP, Clayman RV. Evaluation of synchronous twin pulse technique for shock wave lithotripsy: in vivo tissue effects. Urology. 2003;62:964–7. doi: 10.1016/s0090-4295(03)00685-x. [DOI] [PubMed] [Google Scholar]
- 29.Sheir KZ, El-Diasty TA, Ismail AM. Evaluation of a synchronous twin-pulse technique for shock wave lithotripsy: the first prospective clinical study. BJU International. 2005;95:389–93. doi: 10.1111/j.1464-410X.2005.05306.x. [DOI] [PubMed] [Google Scholar]