Figure 9.
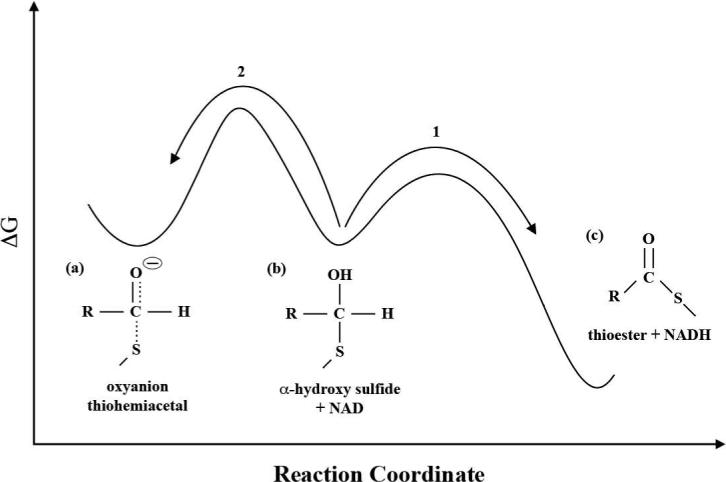
Conceptualized energetics: protonation of the oxyanion thiohemiacetal (a) to form the α-hydroxy sulfide (b) prevents the scission of the S-C bond and formation of the adduct. The hydride transfer reaction (1) must be both kinetically (lower barrier) and thermodynamically more favorable than proton transfer back to the main chain amide (2) with NAD in HT position.
