Abstract
Heterodimerization of G-protein coupled receptors can alter receptor pharmacology. ETA and ETB receptors heterodimerize when co-expressed in heterologous expression lines. We hypothesized that ETA and ETB receptors heterodimerize and pharmacologically interact in vena cava from wild-type (WT) but not ETB receptor deficient (sl/sl) rats. Pharmacological endothelin receptor interaction was assessed by comparing ET-1-induced contraction in rings of rat thoracic aorta and thoracic vena cava from male Sprague Dawley rats under control conditions, ETA receptor blockade (atrasentan, 10 nM), ETB receptor blockade (BQ-788, 100 nM) or ETB receptor desensitization (Sarafotoxin 6c, 100 nM) and ETA plus ETB receptor blockade or ETA receptor blockade plus ETB receptor desensitization. In addition, similar pharmacological ET receptor antagonism experiments were performed in rat thoracic aorta and vena cava from WT and sl/sl rats. ETA but not ETB receptor blockade or ETB receptor desensitization inhibited aortic and venous ET-1-induced contraction. In vena cava but not aorta, when ETB receptors were blocked (BQ-788, 100 nM) or desensitized (S6c, 100 nM), atrasentan caused a greater inhibition of ET-1-induced contraction. Vena cava from WT but not sl/sl rats exhibited similar pharmacological ET receptor interaction. Immunocytochemistry was performed on freshly dissociated aortic and venous vascular smooth muscle cells to determine localization of ETA and ETB receptors. ETA and ETB receptors qualitatively co-localized more strongly to the plasma membrane of aortic compared to venous vascular smooth muscle cells. Our data suggest that pharmacological ETA and ETB receptor interaction may be dependent on the presence of functional ETB receptors and independent of receptor location.
1. Introduction
Veins maintain responsiveness while arteries lose responsiveness to the vasoactive hormone endothelin-1 (ET-1) in situations of exposure to ET-1 [eg. hypertension (Watts et al. 2002) and experimental protocols (Thakali et al. 2004)]. Also, many veins have contractile ETB receptors while most arterial beds do not (Watts et al. 2002; Thakali et al. 2004; Perez-Rivera et al. 2005). Thus, receptors for ET-1 – the G-protein coupled ETA and ETB receptors – may function differently in arteries and veins. Several reports suggest “cross-talk” occurs between ETA and ETB receptors, meaning that activation of one receptor subtype alters the function of the other receptor subtype. For example, in rabbit jugular and saphenous veins and hamster aorta (vessels with contractile ETB receptors), ETA receptor blockade alone did not inhibit ET-1-induced contraction. Only when ETB receptors in these vessels were selectively desensitized with sarafotoxin 6c (S6c), an ETB selective agonist, was ET-1-induced contraction sensitive to ETA receptor blockade. In vessels lacking contractile ETB receptors, like the rat aorta and rabbit carotid artery, ETB receptor desensitization did not alter ETA receptor blockade of ET-1-induced contraction (Lodge et al. 1995). Functional endothelin receptor “cross-talk” or interaction has also been observed in mouse mesenteric veins but not arteries (Perez-Rivera and Galligan 2005), renal afferent but not efferent arterioles (Inscho et al. 2006), and pulmonary arteries (Sauvageau et al. 2006).
While heptahelical receptors canonically interact with G-proteins in a 1:1 ratio, G-protein coupled receptor (GPCR) dimerization (hetero- or homo-) also occurs, potentially affecting pharmacological receptor properties such agonist affinity, potency and efficacy, as well as receptor trafficking and internalization (Bulenger et al. 2005; Maggio et al. 2005; Milligan et al. 2005; Prinster et al. 2005). Human ETA and ETB receptors constitutively heterodimerize when over-expressed in HEK-293 cells (Gregan et al. 2004) and ETA and ETB receptor co-expression in HEK-293 cells is required for trafficking and membrane expression of ETB receptors (Dai and Galligan 2006). Evidence for GPCR dimerization has been well characterized in over-expression systems but there is a paucity of data examining GPCR dimerization in physiologically relevant systems, such as the vasculature. ETB receptor deficient rats were derived from the spotting lethal rat, which carries a natural 301 base pair deletion of the ETB receptor gene encoding the first and second transmembrane domains of the receptor. Since homozygous spotting lethal rats develop aganglionic megacolon and die shortly after birth, the human dopamine-β-hydroxylase promoter was introduced to drive ETB receptor expression primarily in the neonatal enteric nervous system, but also in other catecholaminergic nerves (Gariepy et al, 1996; Gariepy et al, 1998). We hypothesized that ETA and ETB receptors physically interact via receptor heterodimerization in vena cava from wild-type (WT) but not ETB receptor deficient rats and this heterodimerization functionally affects venous ETA and ETB receptor pharmacology.
2. Methods
2.1 Isolated tissue bath protocol
All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals at Michigan State University. Thoracic aorta and vena cava were removed from deeply anesthetized male Sprague-Dawley rats (SD), male homozygous ETB receptor deficient rats (sl/sl) and their male wildtype litter mates (WT) (200-250 g) [pentobarbital (50 mg/kg, i.p.)] and placed in physiological salt solution (PSS) containing (in mM): NaCl, 130; KCl, 4.7; KH2PO4, 1.18; MgSO4 7H2O, 1.17; CaCl2 2H2O, 1.6; NaHCO3, 14.9; dextrose, 5.5; and CaNa2EDTA, 0.03 (pH 7.2). Vessels were cleaned of fat, and rings (3-4 mm) of aorta and vena cava were prepared for measurement of isometric tension as described previously (Thakali et al. 2006). Briefly, rings of aorta and vena cava were placed between two wire hooks; one hook was attached to a stationary glass rod, the other was connected to a force transducer for measurement of isometric contraction. Passive tension was pulled (aorta: 4000 mg; vena cava: 1000 mg) and vessels were equilibrated for one hour in warmed (37°C), aerated (95% O2, 5% CO2) PSS, with frequent buffer changes. Tissue viability was assessed by contraction to an adrenergic agonist (aorta: phenylephrine, 10 μM; vena cava: norepinephrine, 10 μM). Norepinephrine was used to contract vena cava because phenylephrine did not reproducibly contract vena cava and phenylephrine was used to contract aorta such that comparisons to past experiments could be made. Endothelial integrity was confirmed by greater than 80% relaxation to acetylcholine (1 μM) in aorta contracted with phenylephrine (10 nM) and vena cava contraction with norepinephrine (10 μM).
2.1.1
In receptor desensitization studies, vessels were incubated with vehicle (water), ETA receptor antagonist (atrasentan, 10 nM) plus vehicle, ETB receptor agonist (S6c, 100 nM) or ETA receptor antagonist plus ETB receptor agonist (atrasentan + S6c) for one hour and then cumulative concentration response curves to ET-1 (10 pM – 100 nM) were performed. To confirm that the ETB receptor desensitization protocol actually desensitized ETB receptors, vena cava were incubated with S6c (100 nM) for one hour without washing and then challenged again with S6c (100 nM) or norepinephrine (10 μM).
2.1.2
In receptor antagonism studies, vessels were incubated with vehicle (0.0001% DMSO), ETA receptor antagonist (atrasentan, 10 nM) plus vehicle, BQ-788 (100 nM, ETB receptor antagonist, solubilized in vehicle), or ETA plus ETB receptor antagonists [atrasentan (10 nM) + BQ-788 (100 nM)] for one hour, and cumulative concentration response curves to ET-1 (10 pM - 100 nM) were performed. The selective ETA receptor antagonist atrasentan, also known as ABT627, binds ETA and ETB receptors with an IC50 of 0.055 nM and 84.8 nM, respectively (Wu-Wong et al, 2002), while the ETB selective antagonist BQ788 binds ETA and ETB receptors with an IC50 of 1300 nM and 1.2 nM, respectively (Ishikawa et al, 1994). The concentrations of atrasentan (10 nM) and BQ788 (100 nM) were chosen to selectively block ETA and ETB receptors, respectively.
2.2 Western blot analysis
Rat thoracic aorta and vena cava were isolated, dissected, cleaned and then snap-frozen in liquid nitrogen. Vessels were homogenized and protein isolated as previously described (Watts et al. 2002). Fifty micrograms of total protein were loaded on 10% SDS-polyacrylamide gels. After electrophoresis, proteins were transferred to PVDF, membranes blocked in 5% milk overnight and incubated with anti-ETB receptor antibody (1:200 in 5% milk + 0.025% sodium azide, Alomone Labs) overnight. After rinsing blots in tris-buffered saline (+0.5% Tween-20), blots were incubated with an anti-rabbit secondary antibody (1:1000) and developed using standard chemiluminescence protocols.
2.3 Dissociation of vascular smooth muscle cells
Rat thoracic aorta and vena cava were isolated, dissected and cleaned in chilled dissociation solution containing (in mM): NaCl, 136; MgCl2, 1; Na2HPO4, 0.42; NaH2PO4, 0.43; NaHCO3, 4.2; HEPES, 10; sodium nitroprusside, 8.72 and bovine serum albumin, 1 mg/mL (pH 7.4 with NaOH). The entire vena cava and a 4-5 millimeter ring of aorta were cut into small pieces and equilibrated at room temperature for 10 minutes in fresh dissociation solution. Vessels were then incubated in an enzymatic solution (dissolved in dissociation solution) containing papain (26 U/mL) and dithiothreitol (1 mg/mL) (45 minutes with shaking at 37°C). Then vessels were incubated in a second enzymatic solution (dissolved in dissociation solution) containing collagenase (1.95 U/mL), elastase (0.15 mg/mL) and soybean trypsin inhibitor (1 mg/mL) (aorta: 35 minutes; vena cava: 45 minutes with shaking at 37°C). The digestion solution was carefully pulled off (leaving the tissue and cells in the tube) and fresh, cold dissociation solution was added. Cells were placed on ice for five minutes, the dissociation solution was discarded and cells were rinsed again with fresh, cold dissociation solution. The second wash of dissociation solution was gently pipetted off and cells were suspended and triturated (forcefully pipetted approximately 10 times) in OptiMEM (Invitrogen) (plus sodium nitroprusside, 872 nM) to dissociate vascular smooth muscle cells from the blood vessel matrix.
2.4 Immunocytochemistry in freshly dissociated vascular smooth muscle cells
Two hundred microliters of freshly dissociated vascular smooth muscle cells (in OptiMEM) were placed on poly-lysine (50 μg/mL) coated coverslips (12 mm) and allowed to adhere for 45 minutes (37°C, 4% CO2). Some cells were stimulated with ET-1 (100 nM), which was added while cells were adhering to coverslips. Cells were fixed in Zamboni's fixative (20 minutes, room temperature), permeabilized with Triton-X 100 (0.5%, 20 minutes) and incubated with ImageiT signal enhancer (30 minutes, 37°C, Invitrogen). Coverslips were incubated with primary antibodies (ETA: anti-sheep, Fitzgerald Industries; ETB: anti-rabbit, Alomone Laboratories; pan-cadherin: anti-mouse, Sigma; 1:200 dilution in phosphate buffered saline, 0.5% Triton-X 100) for 2 hours (37°C). Coverslips were then incubated with secondary antibodies (Alexa555 anti-rabbit, 1:200; Alexa488 anti-sheep, 1:200; Alexa633 anti-mouse, 1:200; Invitrogen) for 1 hour (37°C). Coverslips were mounted on slides with ProFound anti-fade mounting media (Invitrogen). Confocal images (stacks of 6 micron slices, image resolution = 512×512 pixels) were captured at the Center for Advanced Microscopy at Michigan State University on a Zeiss confocal microscope.
2.5 Data analysis and statistical procedures
Contractility data are presented as mean ± standard error of the mean as a percentage of the initial response to PE/NE (10 μM) for the number of vessels (N) in parentheses. Agonist EC50 values (representing the logarithm of agonist concentration required to cause half-maximal contraction) were calculated using a nonlinear regression analysis (GraphPad Prism, San Diego, CA). When clear maximal responses were not obtained, EC50 values were considered estimates with the true EC50 value being equal to or greater than the calculated value. When comparing two groups, the appropriate Student's t-test was used and when comparing three or more groups, one-way ANOVA and Bonferroni's post-hoc test was performed. In all cases, a P value less than or equal to 0.05 was considered statistically significant. Immunocytochemical analysis of receptor co-localization was qualitatively determined because though confocal imaging parameters were kept constant, experiments were performed on separate days, giving an inherent variability in fluorescence intensity.
2.6 Chemicals
Phenylephrine and norepinephrine were solubilized in water and purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). ET-1 and S6c were obtained from Peninsula Laboratories (Belmont, CA, U.S.A.) and solubilized in deionized water. BQ-788 was purchased from Peninsula Laboratories (Belmont, CA, U.S.A.) and was solubilized in dimethylsulfoxide (DMSO). Atrasentan (DMSO) was a gift from Abbott Laboratories.
3. Results
3.1 Functional endothelin receptor interaction occurs in vena cava but not aorta
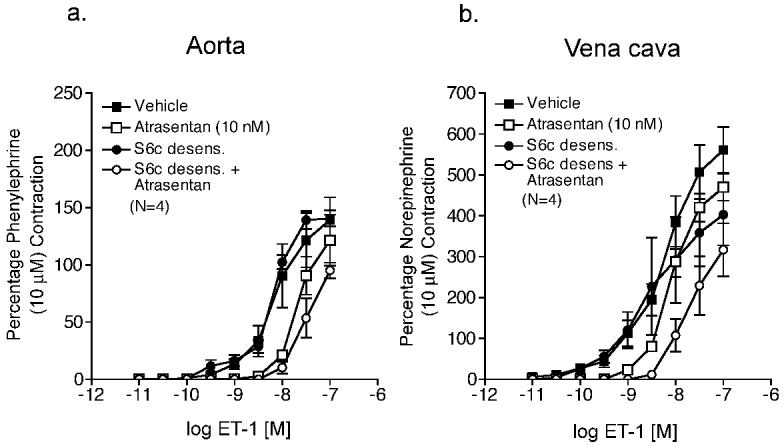
To establish an experimental protocol for examining functional endothelin receptor interaction, ETA receptor blockade (atrasentan, 10 nM) was compared when ETB receptors were unbound (control) or agonist-bound (S6c-desensitized) (results summarized in Table 1) in thoracic aorta and vena cava from male Sprague Dawley rats, as negative and positive examples of functional receptor interaction, respectively. In vena cava, a 1-hour S6c (100 nM) incubation prevented constriction to an additional S6c (100 nM) challenge, but not constriction to norepinephrine (10 μM) (Fig 1), confirming that this protocol desensitized ETB receptors without reducing vascular reactivity to other agonists. Aorta did not contract to S6c (100 nM). In aorta with unbound ETB receptors (control) compared to aorta with agonist bound ETB receptors (S6c-desensitized), ETA receptor antagonism (atrasentan, 10 nM) of ET-1-induced contraction was not different from aorta with agonist-bound ETB receptors (S6c-desensitized) (Fig 2a, Table 1). In vena cava with agonist-bound ETB receptors (S6c-desensitized) compared to vena cava with unbound ETB receptors (control), ETA receptor blockade caused a significantly greater rightward shift in ET-1-induced contraction (control vena cava: no rightward shift in estimated EC50 values; S6c-desensitized vena cava: 3.4-fold rightward shift) (Fig 2b, Table 1).
Table 1.
Maximum contraction and estimated EC50 values for ET-1 (10 pM – 100 nM) - induced contraction in rat thoracic aorta and vena cava under control conditions, ETA receptor blockade, ETB receptor desensitization and ETA blockade plus ETB receptor desensitization.
| Aorta | Vena cava | |||
|---|---|---|---|---|
| Treatment | Max contraction [% PE (10 μM)] |
EC50 (nM) |
Max contraction [% NE (10 μM)] |
EC50 (nM) |
| Vehicle | 139±20 | 6.95±2.13 † | 561±56 | 10.58±4.84 † |
| Atrasentan (10 nM) | 122±22 | 19.90±1.12* † | 470±33 | 8.15±0.60 † |
| S6c (100 nM) | 141±7 | 7.74±1.60* | 403±75* | 6.68±1.90 |
| Atrasentan (10 nM) + S6c (100 nM) |
102±8* † | 32.15±13.25* † | 319±63* | 22.45±4.70* † |
Data are represented as mean ± S.E.M. Atrasentan, ETA receptor antagonist; NE, norepinephrine; PE, phenylephrine; S6c, (sarafotoxin 6c) ETB receptor agonist.
represents a statistically significant difference from control (p<0.05)
represents a statistically significant difference from S6c (p<0.05).
Figure 1.
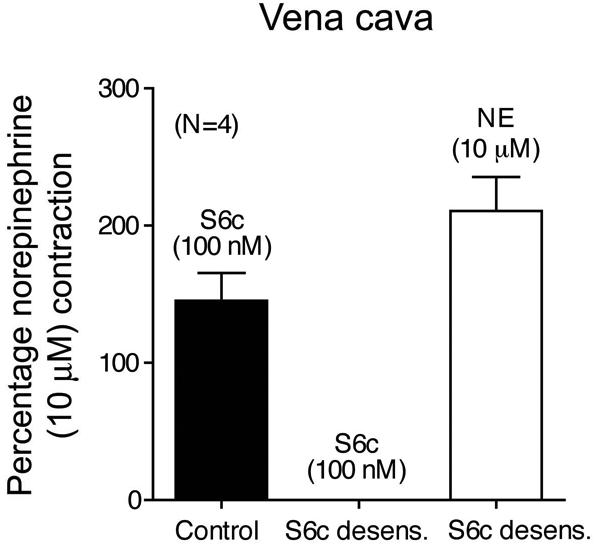
S6c (100 nM) incubation for one hour desensitizes venous ETB receptors, but does not alter the response to NE (10 μM). Vena cava were challenged with S6c (100 nM) and then one hour later were challenged with NE (10 μM) or S6c (100 nM). Data are represented as means ± S.E.M. for the number (N) of animals in parentheses. NE, norepinephrine; S6c, sarafotoxin 6c.
Figure 2.
S6c (100 nM) desensitization augments ETA receptor blockade of ET-1-induced contraction in veins but not arteries. ET-1-induced contraction of aorta (a) and vena cava (b) incubated with vehicle, atrasentan (10 nM), S6c (100 nM), or S6c (100 nM) plus atrasentan (10 nM). Data are represented as means ± S.E.M. for the number (N) of animals in parentheses. S6c, sarafotoxin 6c.
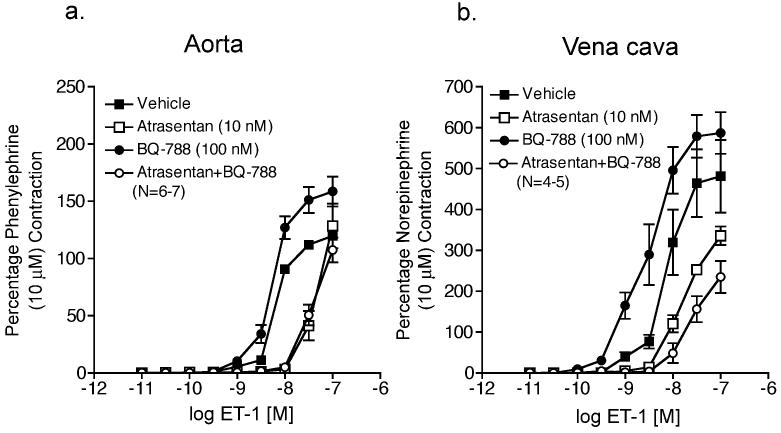
As another way of examining functional ET receptor interaction, cumulative ET-1 concentration response curves were performed in thoracic aorta and vena cava from male Sprague Dawley rats in the presence of vehicle (0.001% DMSO), ETA receptor blockade (atrasentan, 10 nM), ETB receptor blockade (BQ-788, 100 nM) or ETA plus ETB receptor blockade (atrasentan plus BQ-788) (results summarized in Table 2). In aorta with and without ETB receptor blockade, atrasentan caused a similar inhibition of ET-1-induced contraction (Fig 3a). In vena cava without ETB receptor blockade, atrasentan caused a 1.7-fold rightward shift in estimated EC50 values that was significantly less than the 11.1-fold rightward shift induced by atrasentan in the presence of ETB blockade (Fig 3b).
Table 2.
Maximum contraction and estimated EC50 values for ET-1 (10 pM – 100 nM) - induced contraction in rat thoracic aorta and vena cava under control conditions, ETA receptor blockade, ETB receptor blockade and ETA plus ETB receptor blockade.
| Aorta | Vena cava | |||
|---|---|---|---|---|
| Treatment | Max contraction [% PE (10 μM)] |
EC50 (nM) |
Max contraction [% NE (10 μM)] |
EC50 (nM) |
| Vehicle | 120±7 | 7.32±0.70 | 481±89 | 9.70±2.66 † |
| Atrasentan (10 nM) |
128±19 † | 53.12±20.19* † | 336±28* † | 16.84±3.02* † |
| BQ-788 (100 nM) | 158±13* | 4.97±0.78* | 587±51 | 3.76±1.17* |
| Atrasentan (10 nM) + BQ-788 (100 nM) |
107±11 † | 52.84±12.03* † | 235±39* † | 41.71±15.12* † |
Data are represented as mean ± S.E.M. Atrasentan, ETA receptor antagonist; BQ-788, ETB receptor antagonist; NE, norepinephrine; PE, phenylephrine.
represents a statistically significant difference from control (p<0.05)
represents a statistically significant difference from BQ-788 (p<0.05).
Figure 3.
ETB receptor blockade enhances ETA receptor blockade of ET-1-induced contraction in vena cava but not aorta. ET-1-induced contraction of aorta (a) and vena cava (b) incubated with vehicle, atrasentan (10 nM), BQ788 (100 nM), or atrasentan (10 nM) plus BQ788 (100 nM). Data are represented as means ± S.E.M. for the number (N) of animals in parentheses.
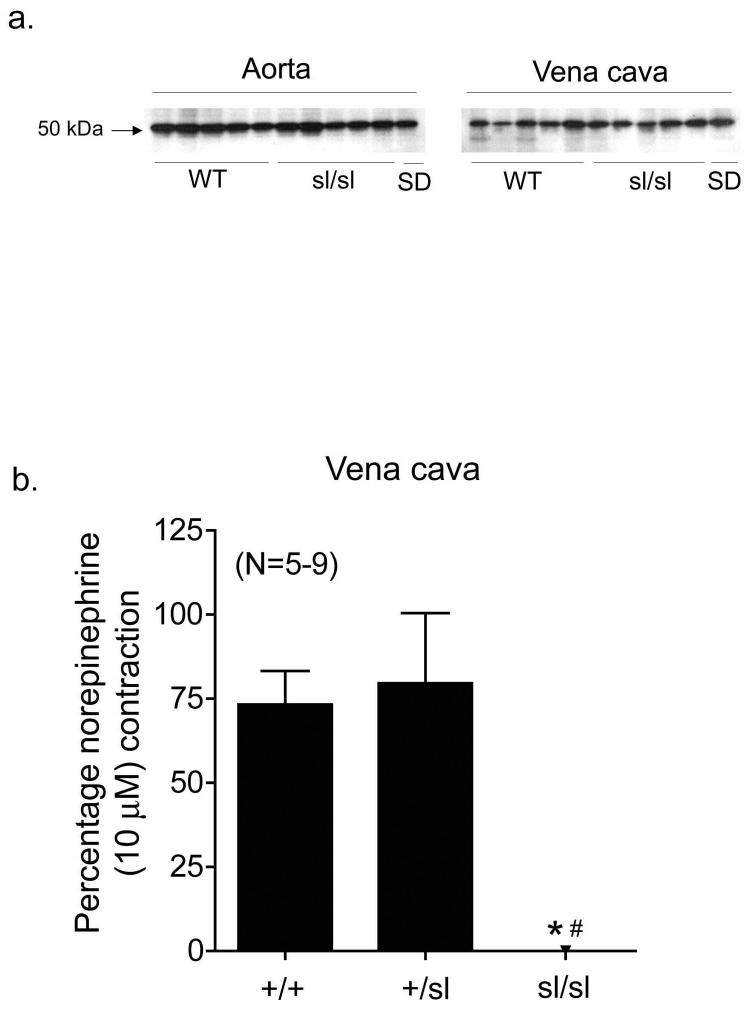
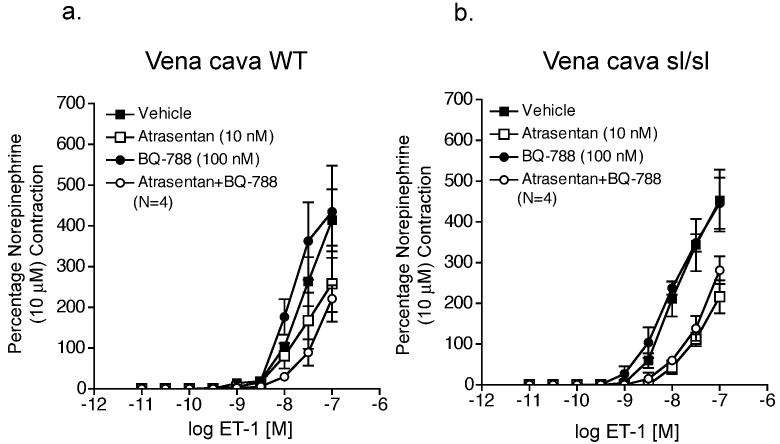
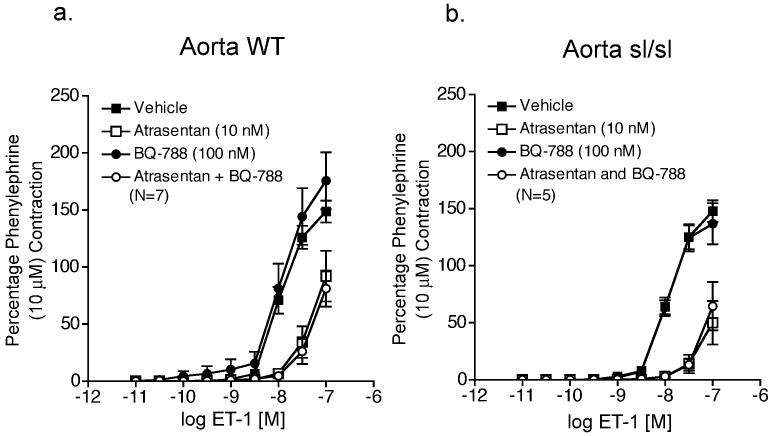
To further demonstrate a critical role for functional ETB receptors in pharmacological endothelin receptor interaction, we compared ETA receptor inhibition of ET-1-induced contraction in aorta and vena cava from male homozygous ETB receptor deficient rats (sl/sl) and their male wild-type littermates (WT) (results summarized in Tables 3 and 4, respectively). Western blot analysis of ETB receptor protein expression demonstrates that aorta and vena cava from both WT and sl/sl rats express ETB receptors (Fig 4a). We confirmed that vena cava from sl/sl rats lacked functional ETB receptors by lack of contraction to the ETB receptor agonist S6c (100 nM) (Figure 4b), while venous norepinephrine (10 μM)-induced contraction was not different from WT and sl/sl rats (WT: 142 ± 22 mg; sl/sl: 103 ± 4 mg). In vena cava (Fig 5a) but not aorta (Fig 6a) from WT rats, ETA receptors were more sensitive to receptor blockade when ETB receptors were blocked (BQ788, 100 nM) (rightward shift in ET-induced contraction - WT vena cava: atrasentan, 1.1-fold; atrasentan plus BQ788, 2.4 fold). In both vena cava and aorta from sl/sl rats (Fig 5b and 6b, respectively), the presence or absence of ETB receptor blockade had no effect on efficiency of ETA receptor blockade (rightward shift in ET-induced contraction – sl/sl vena cava: atrasentan, 2.1-fold; atrasentan plus BQ788, 1.1-fold; sl/sl aorta: atrasentan, 3.7-fold; atrasentan plus BQ788, 4.4-fold).
Table 3.
Maximum contraction and estimated EC50 values for ET-1 (10 pM – 100 nM) - induced contraction in thoracic aorta from WT and sl/sl rats under control conditions, ETA receptor blockade, ETB receptor blockade and ETA plus ETB receptor blockade.
| Aorta WT | Aorta sl/sl | |||
|---|---|---|---|---|
| Treatment | Max contraction [% PE (10 μM)] |
EC50 (nM) |
Max contraction [% PE (10 μM)] |
EC50 (nM) |
| Vehicle | 149±10 | 11.70±1.31 | 148±9 | 12.36±1.23 |
| Atrasentan (10 nM) | 92±22* † | 44.00±0.5* † | 50±19* † | 45.73±2.27* † |
| BQ-788 (100 nM) | 176±25 | 13.51±2.26 | 137±18 | 10.97±1.00 |
| Atrasentan (10 nM) + BQ-788 (100 nM) |
81±16* † | 45.68±3.72* † | 65±21* † | 48.56±0.83* † |
Data are represented as mean ± S.E.M. Atrasentan, ETA receptor antagonist; BQ-788, ETB receptor antagonist; PE, phenylephrine; WT, homozygous wildtype rats; sl/sl, homozygous ETB receptor deficient rats.
represents a statistically significant difference from control (p<0.05)
represents a statistically significant difference from BQ-788 (p<0.05).
Table 4.
Maximum contraction and estimated EC50 values for ET-1 (10 pM – 100 nM) - induced contraction in thoracic vena cava from WT and sl/sl rats under control conditions, ETA receptor blockade, ETB receptor blockade and ETA plus ETB receptor blockade.
| Vena cava WT | Vena cava sl/sl | |||
|---|---|---|---|---|
| Treatment | Max contraction [% PE (10 μM)] |
EC50 (nM) |
Max contraction [% NE (10 μM)] |
EC50 (nM) |
| Vehicle | 414±76 | 16.98±4.24 | 452±76 | 10.82±2.93 |
| Atrasentan (10 nM) | 258±93 † | 18.62±2.87 † | 216±41* † | 22.30±5.48* |
| BQ-788 (100 nM) | 443±88 | 12.39±1.55 | 357±46 | 16.10±10.21 |
| Atrasentan (10 nM) + BQ-788 (100 nM) |
221±33* † | 29.12±4.83* † | 206±27* † | 18.20±3.08* |
Data are represented as mean ± S.E.M. Atrasentan, ETA receptor antagonist; BQ-788, ETB receptor antagonist; NE, norepinephrine; WT, homozygous wildtype rats; sl/sl, homozygous ETB receptor deficient rats.
represents a statistically significant difference from control (p<0.05)
represents a statistically significant difference from BQ-788 (p<0.05).
Figure 4.
Aorta and vena cava from WT and sl/sl rats express ETB receptor protein (a). Each lane represents aortic or venous lysates from a different animal. The ETB receptor agonist S6c (100 nM) constricts vena cava from WT and heterozygous (+/sl) rats but not vena cava from sl/sl rats (b). Data are represented as means ± S.E.M. for the number (N) of animals in parentheses. S6c, sarafotoxin 6c; sl/sl, homozygous ETB receptor deficient; SD, Sprague Dawley; WT, wild-type.
Figure 5.
ETB receptor blockade enhanced ETA receptor blockade of ET-1-induced contraction of vena cava from WT but not sl/sl rats. ET-1-induced contraction of vena cava from WT rats (a) and sl/sl rats (b) incubated with vehicle, atrasentan, BQ788 (100 nM), or atrasentan (10 nM) plus BQ788 (100 nM). Data are represented as means ± S.E.M. for the number (N) of animals in parentheses. sl/sl, homozygous ETB receptor deficient; WT, wild-type.
Figure 6.
ETB receptor blockade has no effect on ETA receptor blockade of ET-1-induced contraction of aorta from WT and sl/sl rats. ET-1-induced contraction of aorta from WT rats (a) and sl/sl rats (b) incubated with vehicle, atrasentan, BQ788 (100 nM), or atrasentan (10 nM) plus BQ788 (100 nM). Data are represented as means ± S.E.M. for the number (N) of animals in parentheses. sl/sl, homozygous ETB receptor deficient; WT, wild-type.
3.2 Immunocytochemical localization of ETA and ETB receptors
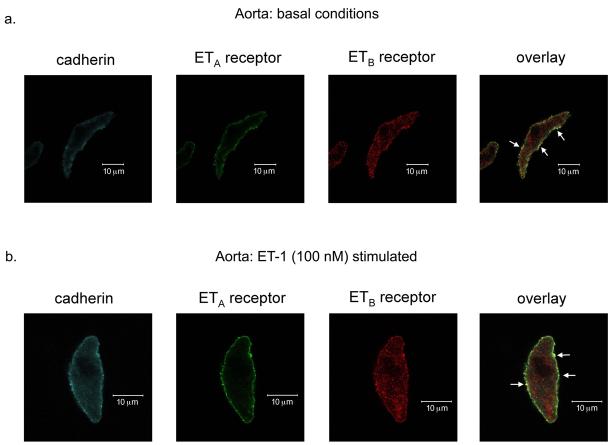
We hypothesized that functional venous ET receptor interaction occurred because ETA and ETB receptors heterodimerize at the plasma membrane of venous vascular smooth muscle cells (VSMCs). We used immunocytochemical analysis to localize ETA and ETB receptors on freshly dissociated aortic and venous VSMCs. Surprisingly, ETA and ETB receptors qualitatively co-localized more strongly with cadherin, a plasma membrane marker, in aortic compared to venous VSMCs under basal conditions (Fig 7a and 8a, respectively). ETB receptors on aortic and venous VSMCs were also present in intracellular compartments, though we did not specifically identify these compartments. ET-1 (100 nM) stimulation (applied when cells were adhering to coverslips) did not induce internalization of either ETA or ETB receptors on the plasma membrane of aortic and venous VSMCs and actually appeared to intensify aortic ETA and ETB receptor co-localization (Fig 7b and 8b, respectively).
Figure 7.
ETA and ETB receptors co-localize to the membrane of aortic vascular smooth muscle cells. Confocal images (6 μm) of ETA receptor, ETB receptor and cadherin (a membrane marker) expression in freshly dissociated aortic vascular smooth muscle cells under basal conditions (a) and after ET-1 (100 nM) stimulation (b). White arrows pointing to yellow on the overlay images represent areas of co-localization of cadherin, ETA receptors and ETB receptors. Images are representative of aortic vascular smooth muscle cells from 5 different rats.
Figure 8.
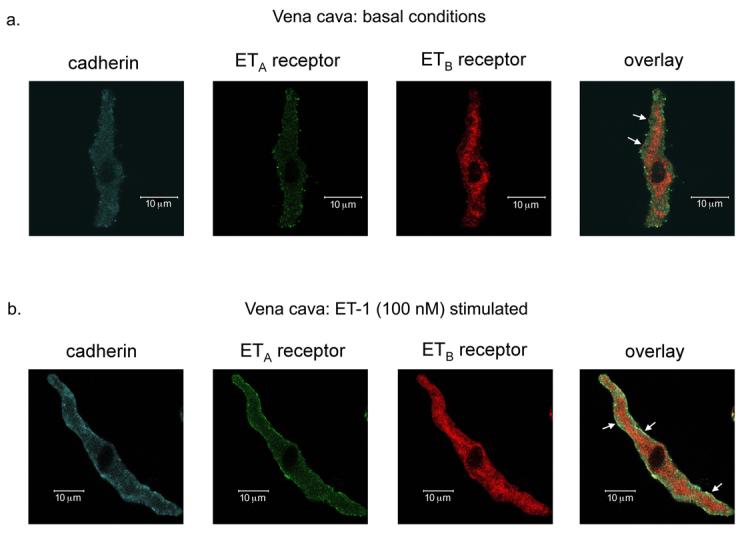
ETA and ETB receptors co-localize to the membrane of venous vascular smooth muscle cells. Confocal images (6 μm) of ETA receptor, ETB receptor and cadherin (a membrane marker) expression in freshly dissociated venous vascular smooth muscle cells under basal conditions (a) and after ET-1 (100 nM) stimulation (b). White arrows pointing to yellow on the overlay images represent areas of co-localization of cadherin, ETA receptors and ETB receptors. Images are representative of venous vascular smooth muscle cells from 4 different rats.
Another technique commonly used to evaluate receptor dimerization is receptor co-immunoprecipitation. Due to technical limitations, we were unable to co-immunoprecipitate ETA and ETB receptors from arterial or venous lysates.
4. Discussion and Conclusions
4.1 Pharmacological ETA and ETB receptor interaction
We hypothesized that ETA and ETB receptors heterodimerize in rat thoracic vena cava from wild-type but not ETB receptor deficient rats, and that this receptor heterodimerization would alter ETA and ETB receptor pharmacology. ETA and ETB receptor heterodimerization provides one reasonable explanation for why veins have a continued response to ET-1 in hypertension, as opposed to arteries, and do not desensitize to the magnitude arteries do when exposed acutely to ET-1. Contractility data comparing ETA receptor function when ETB receptors were desensitized, blocked or non-functional demonstrated that in rat thoracic vena cava, functional ETB receptors were capable of altering ETA receptor pharmacology. Venous ETA receptor blockade inhibited ET-1-induced contraction to a larger degree when ETB receptors were blocked, desensitized or non-functional (because of genetic disruption). Functional ETA and ETB receptor cross-talk occurs in other vessels including rat mesenteric veins (Claing et al. 2002), rat isolated small mesenteric resistance arteries (Mickley et al. 1997), mouse mesenteric veins (Perez-Rivera et al 2005), hamster saphenous and jugular veins (Lodge et al. 1995), pulmonary arteries (Sauvageau et al. 2006), and renal afferent arteries (Inscho et al. 2006). All of these vessels, like the vena cava from wild-type rats, possess contractile ETA and ETB receptors, suggesting that pharmacological ETA and ETB receptor interaction may require the presence of contractile ETB receptors.
We found it surprising that aorta and vena cava from homozygous ETB receptor deficient (sl/sl) rats expressed ETB receptor protein as detected by Western blot analysis. Spotting lethal rats, have a natural deletion of the first exon of the ETB receptor, which encodes for transmembrane domains 1 and 2. Because these rats lack functional ETB receptors during development, they fail to develop an enteric nervous system and die at birth. Gariepy et al (1996 and 1998) created a line of transgenic rats in which a transgene containing the ETB receptor driven by a dopamine β hydroxylase (DβH) promoter was inserted into the DNA of spotting lethal rats. Thus, tissues that express DβH – primarily nerves – also express ETB receptors and the rats develop a normal enteric nervous system. It is possible that the protein detected in our Western blots could represent DβH-driven ETB receptor expression in sympathetic nerves innervating the aorta and vena cava. It is also possible that the mutant ETB receptor, though it lacks 301 base pairs of exon 1, is still transcribed into mRNA and translated into (nonfunctional) protein. The Alomone ETB receptor antibody used in our Western blot experiments targets amino acids 298-314 in the third intracellular loop of the receptor, a region that is not deleted in the mutant ETB receptor. It is also possible that the mutant ETB receptor expressed in sl/sl rats could still physically interact with ETA receptors, as the mutant ETB receptor lacks only the first and second transmembrane domains (Gariepy et al. 1996; Gariepy et al. 1998). However, our pharmacological data demonstrates that though both aorta and vena cava from ETB receptor deficient rats express ETB receptors, these ETB receptors do not couple to contraction, raising the possibility that if ETA and ETB receptors do physically interact, the 1st and 2nd transmembrane domains of the ETB receptor might be important for this interaction.
It is important to note that ETB receptors located on endothelial cells couple to nitric oxide release and vascular relaxation (Hirata et al. 1993). When ET-1 is administered in vivo, endothelial ETB receptors mediate a transient decrease in blood pressure followed by smooth muscle ETA receptor-mediated increase in blood pressure (Kedzierski and Yanagisawa 2001). While we have not observed a biphasic response (transient relaxation followed by a prolonged contraction) to ET-1 in aorta or vena cava when performing cumulative concentration response curves (Watts et al. 2002) or challenge with a bolus (Thakali et al 2004), we cannot exclude the possibility that endothelial ETB receptor blockade alters ET-1-induced contraction. To our knowledge there are no endothelial ETB receptor specific antagonists. We observed that ETA plus ETB receptor blockade caused a greater inhibition or no difference in ET-1-induced contraction compared to ETA receptor blockade alone in vena cava and aorta, respectively, suggesting that in these vessels, endothelial ETB receptors have little effect in modulating ET-1-induced contraction.
4.2 ETA and ETB receptor co-localization in aortic and venous VSMCs
Gregan et al. elegantly demonstrated that ETA and ETB receptors heterodimerize when co-expressed in HEK293 cells, providing the first report that endothelin receptors are included among the myriad of other GPCRs that heterodimerize. Dai et al. (2006) observed that transiently transfected ETA and ETB receptors co-localize at the plasma membrane of HEK cells and that proper plasma membrane expression of ETB receptors requires ETA receptor co-expression. These data support the popular theory that for many pairs of receptor heterodimers, receptor co-expression is a necessary step in receptor processing, maturation and targeting to the plasma membrane (Prinster et al. 2005). Our immunocytochemical experiments demonstrate that both aortic and venous VSMCs express ETA and ETB receptors and that these receptors are present at the plasma membrane. We chose to examine ETA and ETB receptor co-localization in freshly dissociated VSMCs because of the significant autofluorescence in whole vessels sections and because of increased ETB receptor expression in cultured VSMCs (Adner et al. 1998; Moller et al. 2000). Our data suggest that ETA and ETB receptor heterodimerization in vena cava may not account for pharmacological ETA and ETB receptor interaction because ETA and ETB receptors co-localize in both aortic and venous vascular smooth muscle cells and suggest that downstream signaling events mediated by contractile ETB receptors are important in determining pharmacological ETA and ETB receptor interaction.
4.3 Limitations
Receptor dimerization is typically assessed using three methods: 1) fluorescence resonance energy transfer (FRET) to determine receptor proximity, 2) co-immunoprecipitation to determine if the receptors physically interact with each other and, 3) some measure of functional receptor interaction (Angers et al. 2002; Maggio et al. 2005; Milligan and Bouvier 2005; Prinster et al. 2005). One significant limitation of traditional confocal microscopy is that the limit of resolution is approximately 3,000 – 4,000 Å, while FRET allows resolution down to 50 – 100 Å (Wallrabe and Periasamy, 2005). However, the available tools for immunocytochemical analysis of ET receptors in freshly dissociated VSMCs are not powerful enough to make accurate FRET measurements.
We attempted to co-immunoprecipitate ETA and ETB receptors in the lysates of rat thoracic aorta and vena cava to determine if there was a physical interaction between ETA and ETB receptors. Our co-immunoprecipitation experiments were inconclusive because the heavy chain Ig band of the immunoprecipitating antibody resolved at 50 kDa, masking detection immunoprecipitated ET receptors that resolved at the same molecular weight. We tried several commercial kits from Pierce, Santa Cruz and eBioscience designed to eliminate the contaminating Ig bands, but none these kits effectively removed the contaminating Ig band (data not shown). Sauvageau et al. (2006) successfully co-immunoprecipitated ETA and ETB receptors from small pulmonary arteries, but in our hands (and in our samples), these same antibodies used by Sauvageau et al did not specifically bind ETA or ETB receptors and thus were not useful for co-immunoprecipitation. We conclude that like the FRET experiments, the available tools are inadequate for determining if ETA and ETB receptors co-immunoprecipitate in rat thoracic aorta and vena cava.
Most accounts of receptor dimerization have been reported in cell lines over-expressing tagged receptors. These over-expression systems are excellent tools to probe how receptor dimerization occurs, but do not answer the fundamental question: is receptor dimerization a physiological process? A few studies have examined GPCR dimerization in blood vessels and other types of smooth muscle cells. For example, angiotension (AT1) receptors heterodimerize with bradykinin (B2) receptors on human omental vessels and rat renal mesangial cells and increased AT1/B2 receptor heterodimer formation contribute to enhanced angiotensin II reactivity in human preeclampsia and rodent models of hypertension (AbdAlla et al. 2000; AbdAlla et al. 2001; AbdAlla et al. 2005). In cultured airway smooth muscle cells and mouse tracheal rings, prostaglandin EP1 receptors heterodimerize with β2 adrenergic receptors (McGraw et al. 2006) and in aortic vascular smooth muscle cells, prostacyclin receptor and thromboxane A2 receptor heterodimerization determines receptor trafficking (Wilson et al. 2007). However, in most of these studies while functional receptor interaction was observed in smooth muscle cells or blood vessels, direct evidence for receptor dimerization (FRET or co-immunoprecipitation experiments) was determined using over-expression systems. While it is possible that high receptor expression levels in heterologous expression systems compared to natively expressed receptors may artificially induce receptor dimerization, it also possible that the use of epitope-tagged receptors in heterologous expression systems provides a more sensitive method for detection of receptor heterodimers. In vena cava, pharmacological ETA and ETB receptor interaction occurs independently of receptor co-localization, suggesting that interaction between ETA and ETB receptor-activated signal transduction and not receptor heterodimerization mediate functional ETA and ETB receptor interaction.
4.4 Conclusions
Receptor dimerization is a nascent field in receptor characterization and has more recently been recognized as a phenomenon with potential clinical ramifications as many therapeutic drugs target G-protein coupled receptors. Contractility experiments using ETA and ETB receptor antagonists, ETB receptor desensitization and ETB receptor deficient rats demonstrated that pharmacological endothelin receptor requires functional ETB receptors. Our data suggest that functional ETA and ETB receptor interaction in vena cava does not appear to be dependent on receptor co-localization and thus receptor heterodimerization, and may be due to interactions between downstream signaling events. Only with improved tools to assess receptor dimerization, like better ETA and ETB receptor antibodies and novel co-immunoprecipitation approaches, can we actually determine if ETA and ETB receptors heterodimerize when natively expressed in tissues.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant PO1 HL-70687 (to SWW, JJG and GDF) and a Ford Foundation Diversity Fellowship (to KT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci. 2005;26:185–192. doi: 10.1385/JMN:26:2-3:185. [DOI] [PubMed] [Google Scholar]
- Adner M, Uddman E, Cardell LO, Edvinsson L. Regional variation in appearance of vascular contractile endothelin-B receptors following organ culture. Cardiovasc Res. 1998;37:254–262. doi: 10.1016/s0008-6363(97)00206-x. [DOI] [PubMed] [Google Scholar]
- Adner M, Shankley N, Edvinsson L. Evidence that ET-1, but not ET-3 and S6b, ETA-receptor mediated contractions in isolated rat mesenteric arteries are modulated by co-activation of ETB receptors. Br J Pharmacol. 2001;133:927–935. doi: 10.1038/sj.bjp.0704135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci U S A. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Claing A, Shbaklo H, Plante M, Bkaily G, D'Orleans-Juste P. Comparison of the contractile and calcium-increasing properties of platelet-activating factor and endothelin-1 in the rat mesenteric artery and vein. Br J Pharmacol. 2002;135:433–443. doi: 10.1038/sj.bjp.0704441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Galligan JJ. Differential trafficking and desensitization of human ETA and ETB receptors expressed in HEK 293 cells. Exp Biol Med (Maywood) 2006;231:746–751. [PubMed] [Google Scholar]
- Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci U S A. 1996;93:867–872. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 1998;102:1092–1101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inscho EW, Imig JD, Cook AK, Pollock DM. ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol. 2005;146:1019–1026. doi: 10.1038/sj.bjp.0706412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Fukuroda T, Fukami T, Ozaki S, Nagase T, Nishikibe M, Yano M. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci U S A. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- Lodge NJ, Zhang R, Halaka NN, Moreland S. Functional role of endothelin ETA and ETB receptors in venous and arterial smooth muscle. Eur J Pharmacol. 1995;287:279–285. doi: 10.1016/0014-2999(95)00494-7. [DOI] [PubMed] [Google Scholar]
- Maggio R, Novi F, Scarselli M, Corsini GU. The impact of G-protein-coupled receptor hetero-oligomerization on function and pharmacology. FEBS J. 2005;272:2939–2946. doi: 10.1111/j.1742-4658.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, Liggett SB. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley EJ, Gray GA, Webb DJ. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br J Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005;272:2914–2925. doi: 10.1111/j.1742-4658.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- Moller S, Adner M, Edvinsson L. Increased levels of endothelin ETB receptor mRNA in human omental arteries after organ culture: quantification by competitive reverse transcription-polymerase chain reaction. Clin Exp Pharmacol Physiol. 2000;25:788–794. doi: 10.1111/j.1440-1681.1998.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Perez-Rivera AA, Fink GD, Galligan JJ. Vascular reactivity of mesenteric arteries and veins to endothelin-1 in a murine model of high blood pressure. Vascul Pharmacol . 2005;43:1–10. doi: 10.1016/j.vph.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 2005;231:840–846. [PubMed] [Google Scholar]
- Thakali K, Fink GD, Watts SW. Arteries and veins desensitize differently to endothelin. J Cardiovasc Pharmacol. 2004;43:387–393. doi: 10.1097/00005344-200403000-00009. [DOI] [PubMed] [Google Scholar]
- Wallrabe H, Periasamy A. Imaging protein molecules using FRET and FLIM microscopy. Curr Opin Biotechnol. 2005;16:19–27. doi: 10.1016/j.copbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Watts SW, Fink GD, Northcott CA, Galligan JJ. Endothelin-1-induced venous contraction is maintained in DOCA-salt hypertension; studies with receptor agonists. Br J Pharmacol. 2002;137:69–79. doi: 10.1038/sj.bjp.0704831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Dowling JK, Zhao L, Carnish E, Smyth EM. Regulation of thromboxane receptor trafficking through the prostacyclin receptor in vascular smooth muscle cells: role of receptor heterodimerization. Arterioscler Thromb Vasc Biol. 2007;27:290–296. doi: 10.1161/01.ATV.0000252667.53790.4e. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Dixon DB, Chiou WJ, Sorensen BK, Liu G, Jae HS, Tasker A, von Geldern TW, Winn M, Opgenorth TJ. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Clin Sci (Lond) 2002;103:107S–111S. doi: 10.1042/CS103S107S. [DOI] [PubMed] [Google Scholar]