Abstract
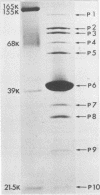
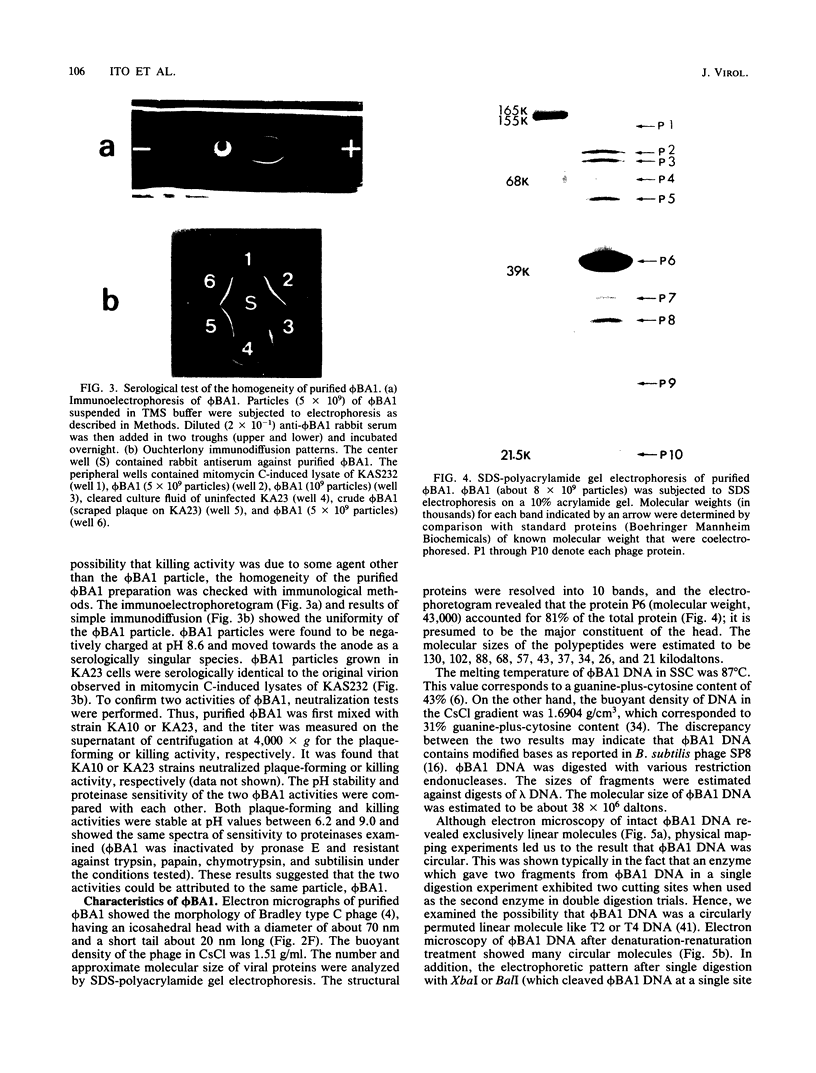
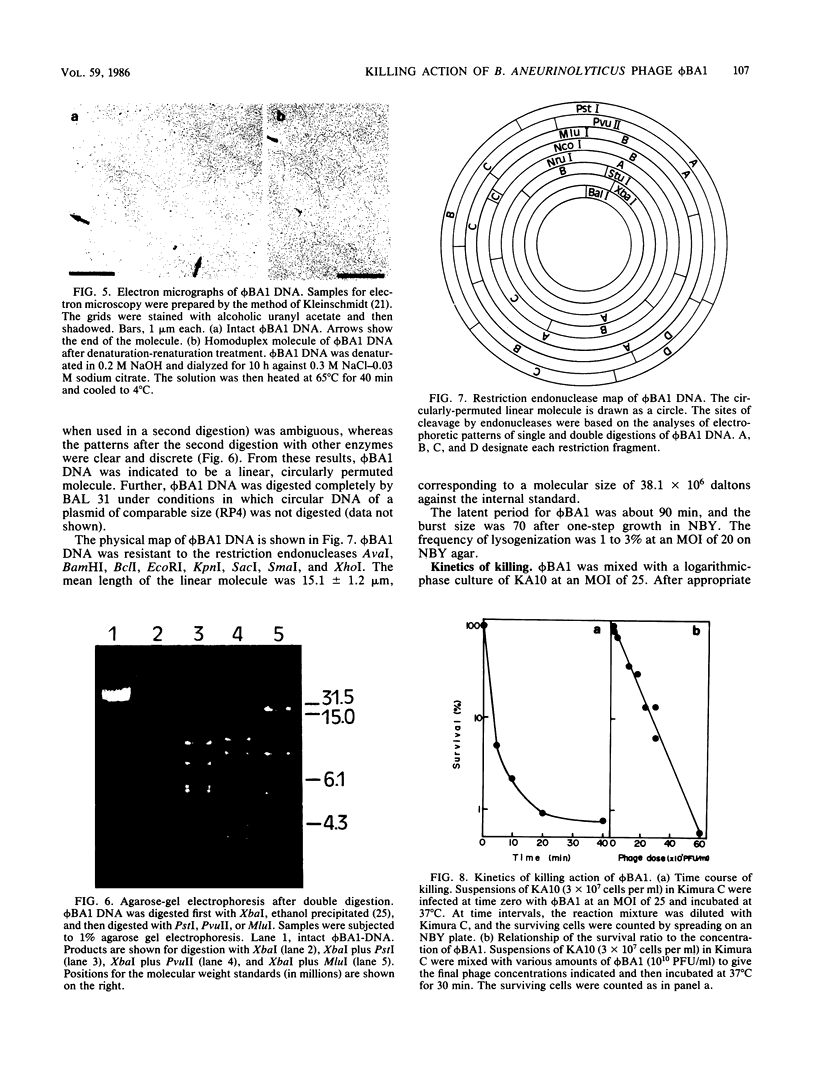
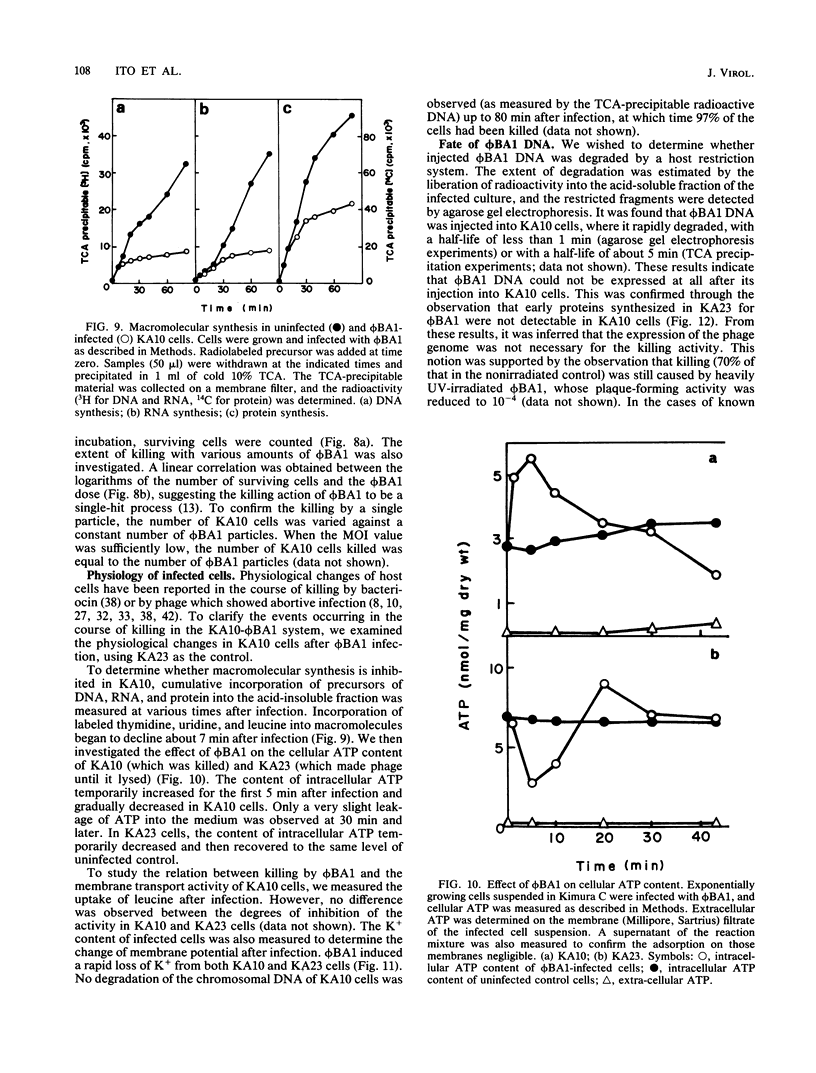
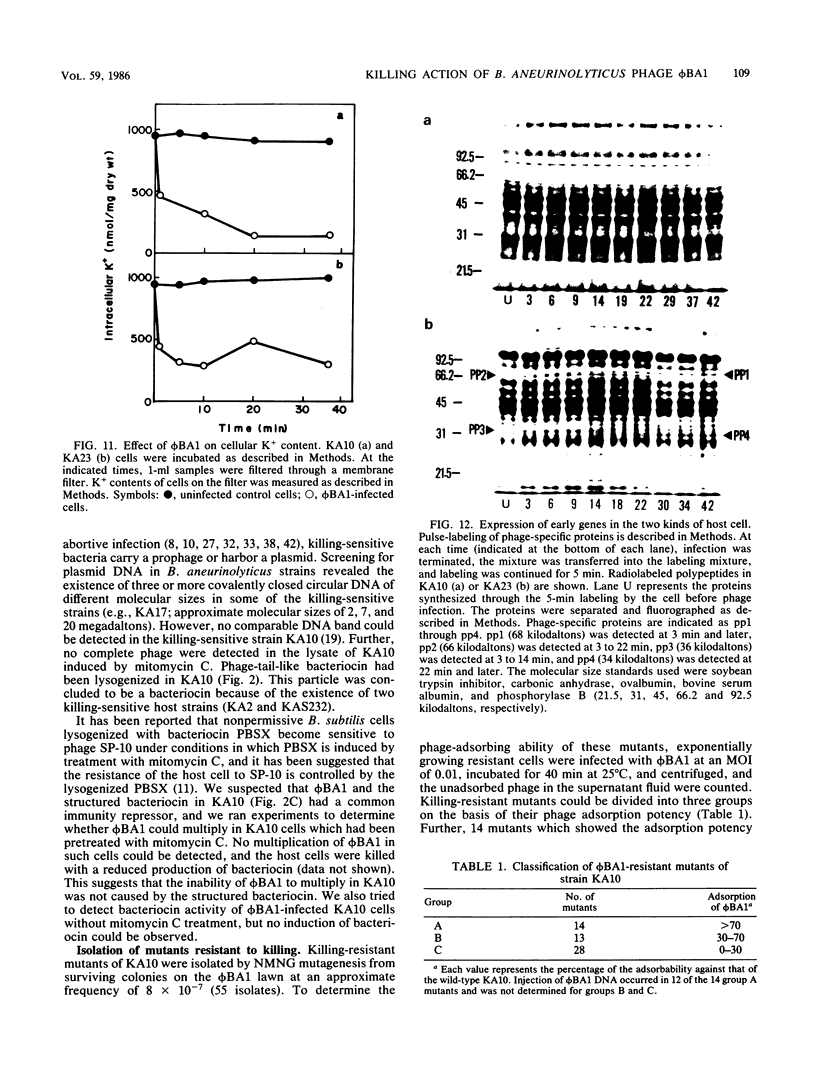
A new temperate phage, phiBA1, was isolated from Bacillus aneurinolyticus, phiBA1 had an icosahedral head with a diameter of about 70 nm and a tail about 20 nm long and contained a circularly permuted, linear duplex DNA of about 38 x 106 daltons. This phage showed two activities: bacteriocin-like killing activity against five strains of B. aneurinolyticus and normal temperate phage activity against three other strains. phiBA1 killed sensitive cells by a single-hit process. After adsorption of phiBA1 to cells sensitive to killing, the content of intracellular ATP increased for the first 5 min and then gradually decreased. Phage DNA injected into the cell immediately after infection was degraded rapidly. Killing was also caused by heavily UV-irradiated phiBA1. Killing-resistant mutants showed normal adsorption of phiBA1 and normal injection of the DNA with its instantaneous restriction. Our results indicate that the killing action of phiBA1 is different from the phenomenon of abortive infection and suggest that the killing might be caused by a proteinaceous component of phiBA1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Kimoto M. Distribution of two types of regular-array particles in the cell wall of Bacillus aneurinolyticus (Kimura et Aoyama). Microbiol Immunol. 1984;28(7):841–846. doi: 10.1111/j.1348-0421.1984.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Hathaway G. M., Rutberg L. Characterization of Temperate Bacillus Bacteriophage phi105. J Virol. 1969 Sep;4(3):264–270. doi: 10.1128/jvi.4.3.264-270.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. K., Duckworth D. H. Membrane damage in abortive infections of colicin Ib-containing Escherichia coli by bacteriophage T5. J Virol. 1977 Jul;23(1):98–105. doi: 10.1128/jvi.23.1.98-105.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Ghisotti D., Zangrossi S., Sironi G. An Escherichia coli gene required for bacteriophage P2-lambda interference. J Virol. 1983 Dec;48(3):616–626. doi: 10.1128/jvi.48.3.616-626.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J., Duckworth D. H. Amino acid and sugar transport in Escherichia coli (ColIb) during abortive infection by bacteriophage T5. J Virol. 1979 May;30(2):421–430. doi: 10.1128/jvi.30.2.421-430.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. D., Bryan T. Productive infection of Bacillus subtilis 168, with bacteriophage SP-10, dependent upon inducing treatments. J Virol. 1968 Aug;2(8):805–812. doi: 10.21236/ad0686354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Dulmage H. T., Carlton B. C. Correlation between specific plasmids and delta-endotoxin production in Bacillus thuringiensis. Plasmid. 1981 May;5(3):352–365. doi: 10.1016/0147-619x(81)90010-x. [DOI] [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- KAGEYAMA M., EGAMI F. On the purification and some properties of a pyocin, a bacteriocin produced by Pseudomonas aeruginosa. Life Sci. 1962 Sep;1:471–476. doi: 10.1016/0024-3205(62)90055-3. [DOI] [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- Kao S. H., McClain W. H. Baseplate protein of bacteriophage T4 with both structural and lytic functions. J Virol. 1980 Apr;34(1):95–103. doi: 10.1128/jvi.34.1.95-103.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keweloh H., Bakker E. P. Permeability changes in the cytoplasmic membrane of Escherichia coli K-12 early after infection with bacteriophage T1. J Bacteriol. 1984 Oct;160(1):347–353. doi: 10.1128/jb.160.1.347-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Shaw A. R., Moody E. E., Hull R. A., Morgan A. F. Is abortive infection by bacteriophage BF23 of Escherichia coli harboring ColIb plasmids due to cell killing by internally liberated colicin Ib? J Virol. 1979 Jul;31(1):31–41. doi: 10.1128/jvi.31.1.31-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Abortive infection of lysogenic Bacillus subtilis 168(SPO2) by bacteriophage phi 1. J Virol. 1974 Apr;13(4):870–880. doi: 10.1128/jvi.13.4.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Rutberg L. Bacteriophage-induced functions in Escherichia coli K (lambda) infected with rII mutants of bacteriophage T4. J Bacteriol. 1966 Jan;91(1):76–80. doi: 10.1128/jb.91.1.76-80.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S. Bactericidal activity of the tail of Pseudomonas aeruginosa bacteriophage PS17. J Virol. 1979 Dec;32(3):958–967. doi: 10.1128/jvi.32.3.958-967.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensma H. Y. Effect of defective phages on the cell membrane of Bacillus subtilis and partial characterization of the phage protein involved in killing. J Gen Virol. 1981 Oct;56(Pt 2):275–286. doi: 10.1099/0022-1317-56-2-275. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Wright A., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology. 1974 Dec;62(2):367–384. doi: 10.1016/0042-6822(74)90399-7. [DOI] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, MACHATTIE L. A. CIRCULAR T2 DNA MOLECULES. Proc Natl Acad Sci U S A. 1964 Nov;52:1297–1301. doi: 10.1073/pnas.52.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Ganesan A. T., Young F. E. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974 Apr;13(4):916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]