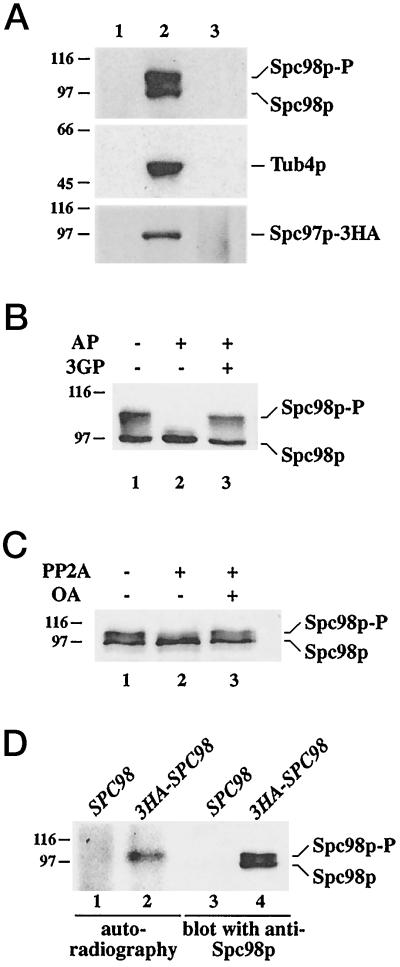
Figure 1.
Immunoprecipitation and phosphorylation of Spc98p. (A) Immunoprecipitation of Tub4p, Spc98p, and Spc97p-3HA is quantitative. Yeast cells were lysed with glass beads in the presence of 1% Triton X-100 as described in MATERIALS AND METHODS. The lysate was centrifuged for 5 min at 500 × g to remove unlysed cells. The supernatant was then centrifuged (10 min, 5,000 × g) to give a pellet (lane 1) and a supernatant. This supernatant was incubated with anti-Tub4p, anti-Spc98p, or anti-HA (Spc97p-3HA) antibodies cross-linked to protein-G Sepharose for 4 h at 4°C. The protein-G beads were collected by centrifugation (lane 2). Proteins in the supernatant were concentrated by trichloroacetic acid (TCA, 10% final concentration) precipitation (lane 3). Comparable aliquots were analyzed by immunoblotting using anti-Tub4p, anti-Spc98p, or anti-HA (Spc97p-3HA) antibodies. (B) Spc98p is a phosphoprotein. Immunoprecipitated Spc98p was incubated without phosphatase (lane 1), or with AP in the absence (lane 2) or in the presence (lane 3) of the inhibitor 3-glycerophosphate (3GP) (100 mM). Spc98p was detected by immunoblotting using goat anti-Spc98p antibodies. (C) Serine or threonine residues of Spc98p are phosphorylated. Spc98p was incubated in buffer only (lane 1), or with the phosphoserine/threonine-specific phosphatase PP2A in the absence (lane 2) or in the presence (lane 3) of 5 μM of the inhibitor okadaic acid (OA). Samples were analyzed by immunoblotting with anti-Spc98p antibodies. (D) In vivo phosphorylation of Spc98p. SPC98 (lanes 1 and 3) and 3HA-SPC98 (lanes 2 and 4) cells were grown in the presence of [32P]-orthophosphate. Immunoprecipitation was performed with anti-HA antibodies. 3HA-Spc98p was visualized by autoradiography (lanes 1 and 2) and immunoblotting with anti-Spc98p antibodies (lanes 3 and 4). The position of the molecular weight standards β-galactosidase (116 kDa) and phosphorylase b (97 kDa) is indicated. Experimental procedures are described in MATERIALS AND METHODS.