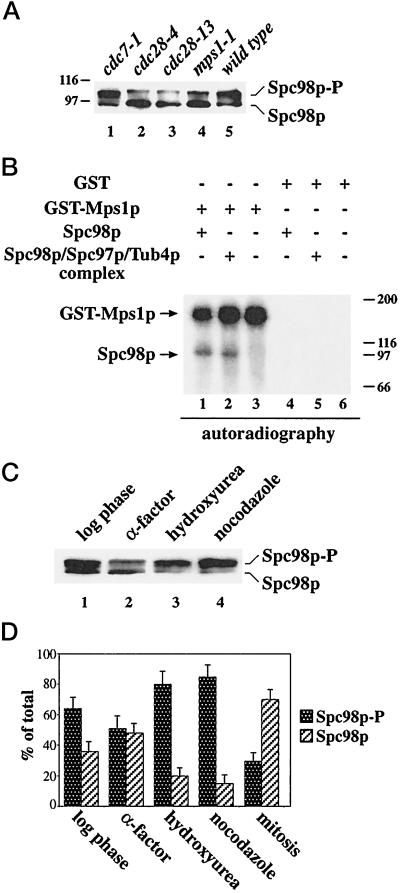
Figure 3.
Spc98p phosphorylation is reduced in cdc28 and mps1–1 cells and increased after checkpoint activation. (A) Cells of cdc7–1 (lane 1), cdc28–4 (lane 2), cdc28–13 (lane 3), mps1–1 (lane 4), and wild type (lane 5) were shifted to 37°C for 4 h. After cell lysis, Spc98p was immunoprecipitated and analyzed by immunoblotting. (B) Mps1p phosphorylates Spc98p in vitro. GST-Mps1p (lanes 1 to 3) or GST (lanes 4 to 6) were isolated from yeast cells using glutathione Sepharose as described (Lauzéet al., 1995). The purified proteins were incubated with buffer (lanes 3 and 6) or immunoprecipitated Spc98p from a strain overexpressing SPC98 (lanes 1 and 4), or SPC98, SPC97, and TUB4 simultaneously (lanes 2 and 5) in the presence of [γ-32P]ATP. Labeled proteins were visualized by autoradiography. Myosin (200 kDa), β-galactosidase (116 kDa), phosphorylase b (97 kDa), and bovine serum albumin (66 kDa) were used as molecular standards. (C) Checkpoint activation increases Spc98p phosphorylation. Spc98p of logarithmic growing cells (lane 1) or of cells treated with α-factor (lane 2), hydroxyurea (lane 3), or nocodazole (lane 4) was immunoprecipitated and then analyzed by immunoblotting. (D) The ratio of phosphorylated to unphosphorylated Spc98p of logarithmic growing cells, or of cells arrested with α-factor, hydroxyurea, or nocodazole, or of mitotic cells of a synchronized culture (see Figure 2A; [t = 70 min]) was quantified by densiometric scanning of the blots using the NIH image program. Shown is the average of three independent experiments. The bars indicate the variation of the experiments.