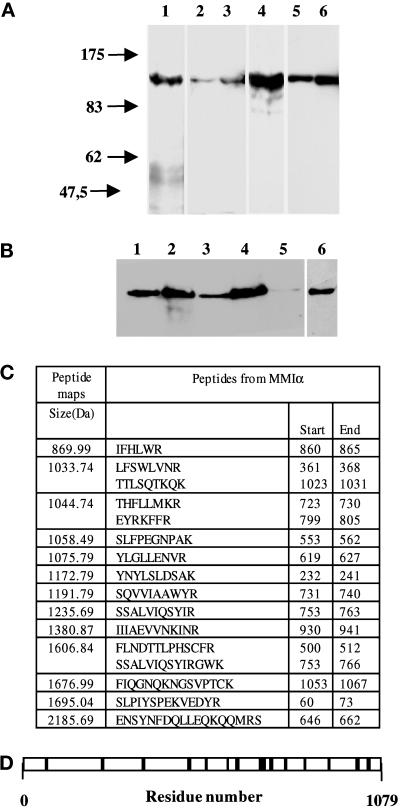
Figure 1.
Antibodies directed against BBMI and Myr 1 recognize MMIα in the BWTG3 cells. (A) Twenty micrograms of proteins from mouse liver homogenate (lanes 1 and 4), total BWTG3 cell homogenate (lanes 2 and 5), and postnuclear supernatant (lanes 3 and 6) were separated by 7% SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were probed with a monoclonal antibody directed against BBMI head domain (CX-1; lanes 1–3), or with anti-Myr 1 antibodies (Tu 30; lanes 4–6). (B) Western blot analysis of BWTG3 cell lysate immunoprecipitated with a monoclonal antibody directed against BBMI head domain (CX-1) or the antibodies directed against Myr 1 (Tu 30). Total cell lysate (lane 1), total cell lysate immunoprecipitate with anti-BBMI antibody (lane 2), supernatant of the cell lysate after the immunoprecipitation with anti-BBMI antibody (lane 3), supernatant immunoprecipitate with anti-Myr 1antibodies (Tu 30; lanes 4 and 6), and supernatant after immunoprecipitations with both antibodies (lane 5) were separated by 7% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with anti-Myr 1 antibodies (Tu 30; lanes 1–5) and with the anti-BBMI antibody (CX-1; lane 6). (C) The peptide map derived from the trypsin proteolysis of the 130-kDa protein immunoprecipitated with anti-Myr 1 was compared with the OWL data base using a Baysien algorithm (Profound). Thirteen peptides matched 16 peptides of MMIα. (D) Distribution of matched peptides along the sequence of MMIα. Note that the majority of the peptides were located on the tail sequence that diverges more from one subclass of myosin I to another.