Figure 5.
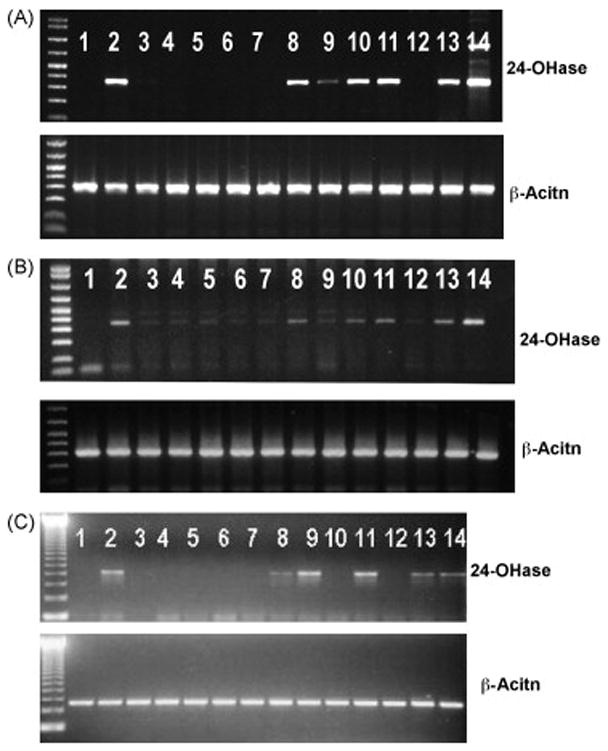
Effects of N-benzyl-1α,25-(OH)2D3-26,23-lactam analogues and N-phenetyl-1α,25-(OH)2D3-26,23-lactam analogues on 25-OH-D3-24-hydroxylase gene expression induced by 1α,25-(OH)2D3 in HL-60 cells (A), human osteoblastic cell line (HOS cells) (B) and primary mouse osteoblastic cells (C). The experimental procedures for gene expression induced by vitamin D analogues were carried out as described in Materials and Methods. 1, vehicle; 2, 10−8M 1α,25-(OH)2D3; 3, 10−6M TEI-9647; 4, 10−6M (23S,25S)-DLAM-1P; 5, 10−6M (23S,25R)-DLAM-1P; 6, 10−6M (23S,25S)-DLAM-2P; 7, 10−6M (23S,25R)-DLAM-2P; 8, 10−6M 1α,25-(OH)2D3-26,23-lactone; 9, 10−8M 1α,25-(OH)2D3 + 10−6M TEI-9647; 10, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25S)-DLAM-1P; 11, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25R)-DLAM-1P; 12, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25S)-DLAM-2P; 13, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25R)-DLAM-2P; 14, 10−8M 1α,25-(OH)2D3 + 10−6M 1α,25-(OH)2D3-26,23-lactone. Similar results were seen in two independent experiments.
