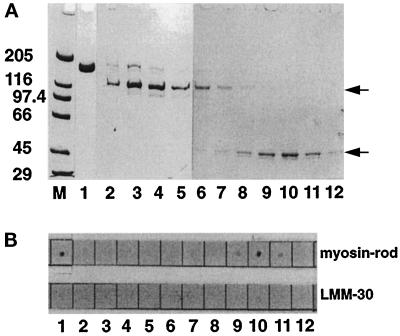
Figure 2.
Binding of M-protein and its proteolytic 45-kDa fragment to myosin rod. (A) SDS-PAGE analysis (4–14%) of M-protein purified from bovine skeletal muscle (lane 1) and gel filtration fractions of an endoproteinase Asp-N digest of M-protein (see MATERIALS AND METHODS) (lanes 2–12). M = molecular mass standards in kilodaltons. (B) M-protein (1) and corresponding fractions of the M-protein digest (2–12) spotted on nitrocellulose filters after incubation with biotinylated myosin-rod or LMM 30. Note that only native M-protein (1) and its 45-kDa fragment (comprising domains Mp2–Mp4; fractions 9–11) show binding to myosin rod, while the proteolytic 110-kDa fragment of M-protein (comprising domains Mp5–Mp13; lanes 2–6) does not bind to myosin rod (the positions of these proteolytic fragments are indicated by arrows). Note also that both M-protein fragments do not bind to LMM30. Thus myosin rod binding of M-protein requires domains Mp2–Mp4.