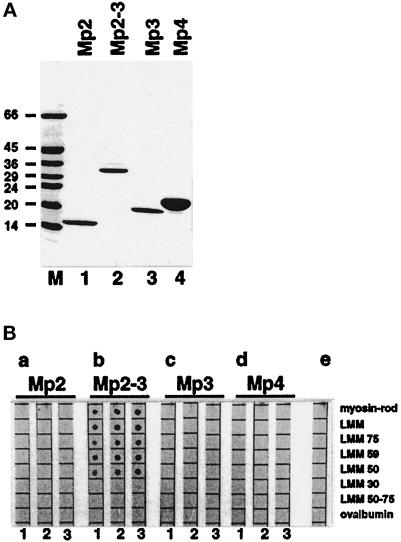
Figure 3.
Binding of recombinant M-protein fragments to proteolytic and recombinant derivatives of myosin. (A) SDS-PAGE analysis (6–20% gradient gels) of purified recombinant M-protein fragments: Mp2 (lane 1), Mp2–Mp3 (lane 2), Mp3 (lane 3), and Mp4 (lane 4). M = molecular mass standards in kilodaltons. For domain structure of M-protein see Figure 1. (B) Results of binding assays. The same amounts (ca. 1 μg) of myosin rod, proteolytic LMM, LMM 75, LMM 59, LMM 50, LMM 30, LMM 50–75, and ovalbumin, serving as a control, were spotted on nitrocellulose filters and overlaid with increasing concentrations (1 = 0.5 μM, 2 = 1.5 μM, 3 = 4.5 μM) of M-protein fragments Mp2 (a), Mp2 to Mp3 (b), Mp3 (c), and Mp4 (d). (e) Control without protein in the overlay buffer. Binding of M-protein fragments carrying the carboxy-terminal EEF-tag was detected with monoclonal antibody YL1/2, which specifically recognizes this tag. Note the specific binding of M-protein fragment Mp2–Mp3 to all myosin fragments that contain the central portion of LMM (myosin heavy chain residues 1506–1674) but not to LMM30 and LMM50–75.