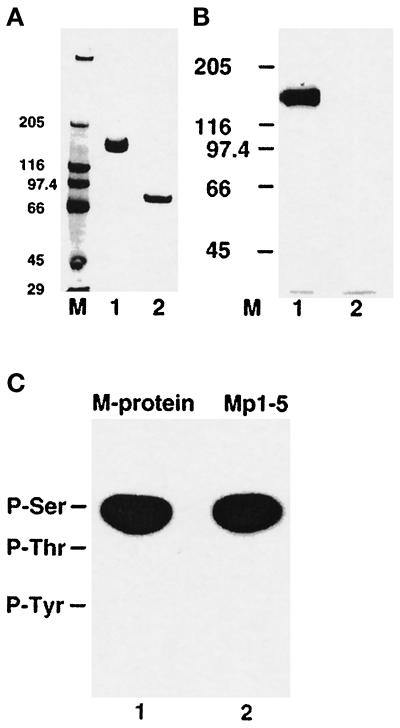
Figure 4.
Phosphorylation of native M-protein and phosphoamino acid analysis of M-protein and a recombinant Mp1–5 fragment. (A) M-protein from bovine skeletal muscle (lane 1) and a limited digest with trypsin (lane 2), analyzed on 4–12% SDS-PAGE (see MATERIALS AND METHODS). (B) The corresponding autoradiograph of the samples shown in panel A after incubation with PKA in the presence of [γ-32P] ATP shows that M-protein (lane 1) is readily phosphorylated while its tryptic fragment (Mp6– Mp13) is not (lane 2). M = molecular mass standards in kilodaltons. (C) Phosphoamino acid analysis of native M-protein from bovine skeletal muscle (lane 1) and the recombinant M-protein fragment Mp1–Mp5 (lane 2) after phosphorylation with PKA. Positions of marker amino acids are indicated. Clearly, phosphorylation occurs in both samples exclusively on serine residues.