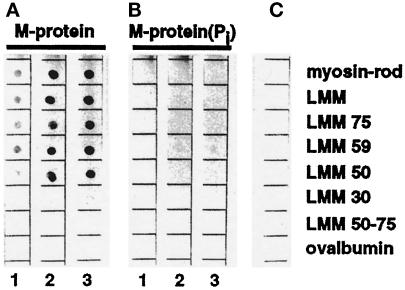
Figure 6.
Interaction of unphosphorylated and PKA phosphorylated M-protein with proteolytic and recombinant derivatives of myosin. The same amounts (1 μg) of myosin-rod, proteolytic LMM, LMM75, LMM59, LMM50, LMM30, LMM50–75, and ovalbumin, serving as a control, were spotted on nitrocellulose filters and overlayed with increasing concentrations (1 = 0.1 μM, 2 = 0.3 μM, 3 = 1.0 μM) of unphosphorylated (a) and phosphorylated (b) M-protein. (c) is a control without protein. Binding of M-protein was detected using the monoclonal M-protein antibody MpAA280 (see Obermann, et al., 1996). Note that phosphorylation of M-protein almost completely abolished binding to myosin derivatives.