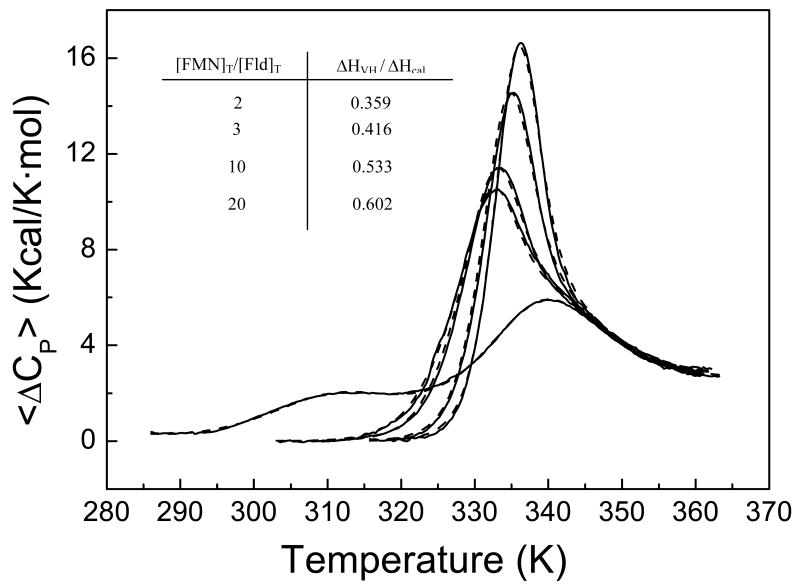
FIGURE 8.
Thermal unfolding of H. pylori flavodoxin at five different total concentrations of FMN: 0, 32.6, 48.9, 163 and 326 μM. The concentration of protein is 16.3 μM. The experiments were made in Tris 165 mM, pH 8.5. In the absence of ligand, the protein exhibits three transitions (the endothermic peak at high temperature is not a single unfolding transition, but the superposition of two close transitions). The transition most affected by the FMN binding is the one taking place at low temperature.