Table 1.
Oxidation of Allylic Esters and Carbonates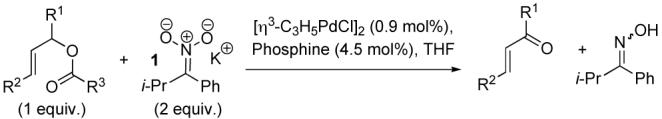
| Entry | Substrate | Product | Phosphine | Yield (time) |
|---|---|---|---|---|
| 1 | 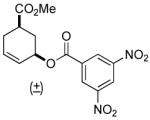 |
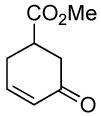 |
PPh3 | 100% (<1 h) |
| 2 | PMePh2 | 95% (<1 h) | ||
| 3 | dppf | 80% (2 h) | ||
| 4 | dppb | 73% (26 h)a | ||
| 5 | 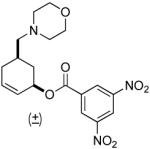 |
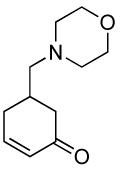 |
PPh3 | 92% (1 h) |
| 6 | 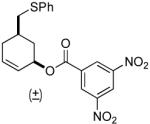 |
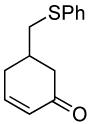 |
PPh3 | 96% (>1 h) |
| 7 | 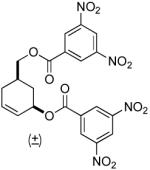 |
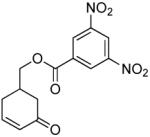 |
PPh3 | 88% (> 1 h) |
| 8 | 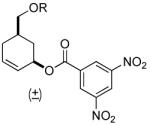 |
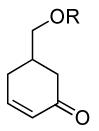 |
PPh3 | R=OTBS, 95% (2 h) |
| 9 | PPh3 | R=OH, 82% (2 h) | ||
| 10 | 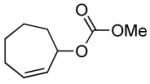 |
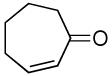 |
PPh3 | 71% (3 h)b |
| 11 | 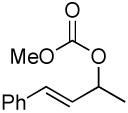 |
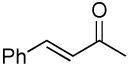 |
PPh3 | 53% (2.5 h) |
| 12 | rac-2 | 97% (4 h) |
95% brsm.
Volatile material. Actual yield may be higher.
