Table 2.
Asymmetric Oxidation of Allylic Esters and Carbonates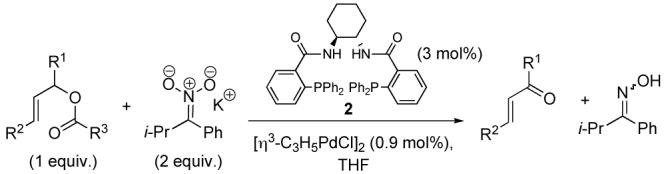
| Entry | Substrate | Product | Yield (brsm) | Time | eec |
|---|---|---|---|---|---|
| 1 a | 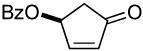 |
61% (73%) | 0.6 h | 99% | |
| 2a | 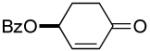 |
75% | 2 h | 98% | |
| 3 | 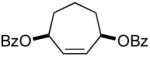 |
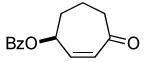 |
45% (95%) | 12 h | 99% |
| 4 a | 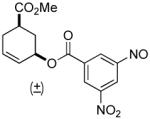 |
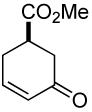 |
80% | 1 h | 98% |
| 5b | 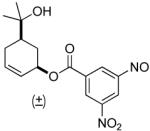 |
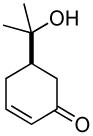 |
77% | 5 h | 98% |
| 6 b | 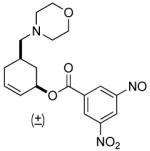 |
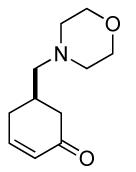 |
68% (98%) | 12 h | 99% |
| 7 b | 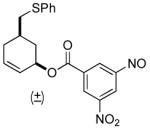 |
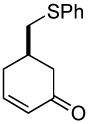 |
69% (81%) | 23 h | 92% |
| 8 b | 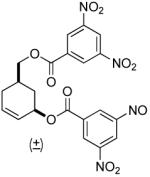 |
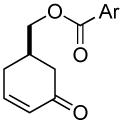 |
81% (99%) | 8 h | 97%d |
| 9 a | 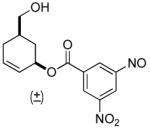 |
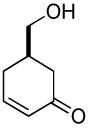 |
63% (74%) | 14 h | 99% |
| 10a, e | 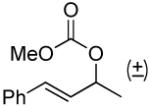 |
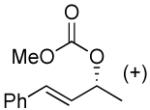 |
49% (98%)f | 12 h | 99% |
absolute stereochemistry confirmed by comparison of optical rotation with known compound.
absolute stereochemistry assigned by analogy.
determined by chiral HPLC or GC analysis.
determined by hydrolysis to alcohol.
1 equiv. of nitronate used.
Based on 50% theoretical yield.
