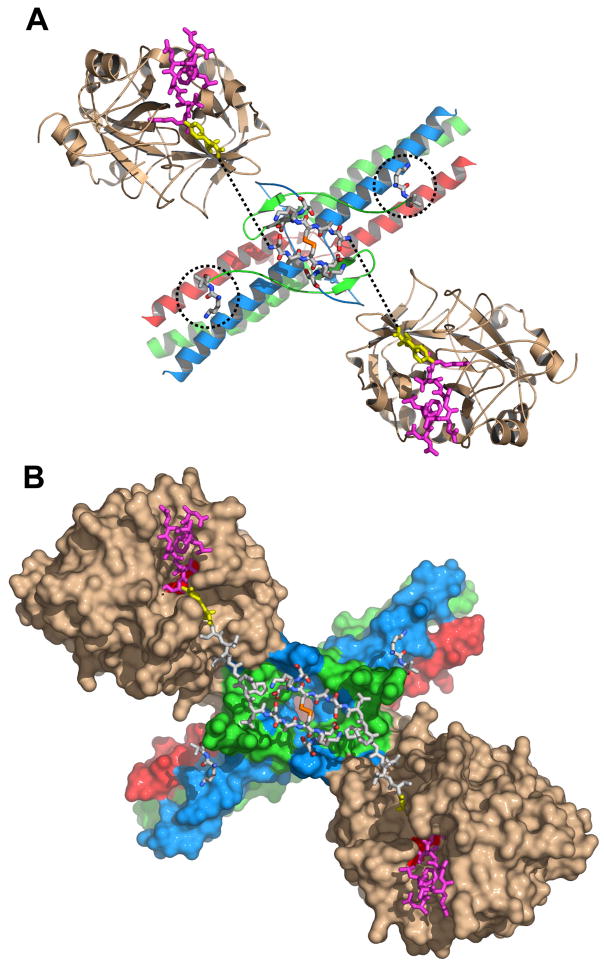
Fig. 2. Arrangement of the NH2-terminal portions of the Aα chains in the structure of the complex of thrombin with the Eht fragment.
Panel A shows a ribbon diagram of the thrombin-Eht complex with the newly built Aα26-31 and Bβ54-55 residues shown by sticks colored by atom types: blue for nitrogens, red for oxygens, orange for sulfurs, and white for carbons; locations of Bβ54-55 residues are also indicated by the dotted circles. The thrombin-bound fpA variant (28) (PDB entry 1UCY) is shown by magenta (Aα7-16) and yellow (Aα17-19) sticks. Dotted lines indicate the distance between AαArg19 and AαSer26 (see text). Panel B illustrates the solvent accessible surface of the complex with the newly modeled Aα20-25 connecting segments shown by white sticks. In both panels the Aα, Bβ and γ chains of Eht are colored in blue, green and red, respectively; thrombin molecules are in beige.