Abstract
(S)-5-Bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-2,3-dimethoxybenzamide (4) has pico-molar in vitro binding affinity to D2 receptor (Ki(D2) = 0.003 nM) with lower affinity to D3 receptor (Ki (D3)= 0.22 nM). In this study, we describe radiosynthesis of [11C]4 and evaluation of its binding characteristics in post mortem human brain autoradiography and with PET in cynomolgus monkeys. The 11C labelled 4 was synthesized by using [11C]methyltriflate in a methylation reaction with its phenolic precursor with good incorporation yield (64 ± 11 %, DCY) and high specific radioactivity >370 GBq/μmol (> 10 000 Ci/mmol). In post mortem human brain autoradiography [11C]4 exhibited high specific binding in brain regions enriched with dopamine D2/D3 receptors and low level of non-specific binding. In cynomolgus monkeys [11C]4 exhibited high brain uptake reaching 4.4% ID at 7.5 minutes. The binding in the extrastriatal low density D2-receptor regions; thalamus and frontal, parietal, temporal and occipital cortex, was clearly visible. Pretreatment with raclopride (1mg/kg as tartrate) caused high reduction of binding in extrastriatal regions, including cerebellum. [11C]4 is a promising radioligand for imaging D2 receptors in low density regions in brain.
Keywords: Dopamine, D2-receptor, antagonist, PET, autoradiography
1. Introduction
Positron emission tomography (PET) has been widely utilized in visualizing striatal and extrastriatal dopamine D2 receptors using D2/D3 receptor antagonists such as [11C]raclopride, [11C]FLB 457 and [18F]fallypride (Figure 1).1–5 These benzamides have provided deep knowledge in understanding involvement of changes in dopamine neurotransmission in development of neuropsychiatric disorders such as Parkinson’s disease, schizophrenia and depression.6–10 Pico-molar binding affinity D2/D3 receptor ligands [11C]FLB 457 and [18F]fallypride have been used in exploring changes in D2/D3 receptor densities especially in extrastriatal structures in the brain, for which [11C]raclopride as a moderate affinity ligand does not provide enough signal for quantitative D2/D3 receptor imaging.
Figure 1.
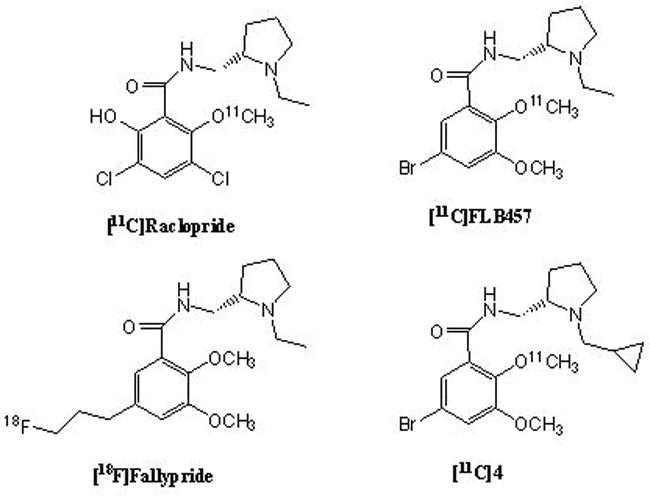
Chemical structures of some commonly used D2/D3-receptor tracers for PET and structure of the new [11C]cyclopropylmethyl analogue of FLB 457 ([11C]4).
In extrastriatal D2-receptor regions, i.e. thalamus, hypothalamus, amygdala, substantia nigra, frontal cortex, and temporal cortex, D2 receptor density is ten to hundred times lower than in the high density striatum (measured with D2/D3 antagonist),11 which sets high demands for selectivity and binding affinity of the radioligand. The generally used D2 receptor antagonists have almost equal affinity to both D2 and D3 receptors and their usage in D2 receptor imaging has been promoted because of the low receptor density of D3 receptor in the brain, and because of the higher occupancy of D3 receptors in vivo by endogenous dopamine.12 However, the recent advancement of D3 receptor preferring radioligands has provided new information on the brain distribution of the receptor and the role of these receptors in drug addiction and in neurological and neuropsychiatric disorders.13–15 While located primarily in limbic brain regions, moderate densities of D3 receptors are expressed also in thalamus, hypothalamus and nucleus accumbens, regions overlapping with the low density D2 regions. There are recent findings that D2 and D3 receptors have a diverse role in progress of schizophrenia, Parkinson’s disease and in drug abuse.16–18 For discriminating their role in these pathological conditions, radioligands purely selective only to one receptor type would be beneficial.
The new cyclopropylmethyl analogue of FLB 457, (S)-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-5-bromo-2,3-dimethoxybenzamide (4), was discovered in studies, which focused on development of D3 receptor selective substituted benzamide antagonists.19 In the structural series, effect of cyclopropylmethyl group and substitutions in the aromatic and pyrrolidine ring were investigated. On the contrary to the expectations, the compound 4 was found to have ten times higher in vitro binding affinity to D2 receptor than the widely used structural analogues FLB 457 and fallypride, but with a similar affinity to D3 receptor (Ki(D2) = 0.003 nM, Ki (D3)= 0.22 nM, ratio KiD2/D3 = 0.014.19 Despite its early discovery, it has not been radiolabeled and evaluated in vivo before. Our aim was to radiolabel the new D2 receptor selective analogue compound 4, and preliminary evaluate its binding characteristics in post mortem human brain and in vivo in cynomolgus monkeys.
2. Chemistry
2.1. Synthesis of the precursor
Benzotriazol-1-yloxy-tris(dimethylamino)phosphonium hexafluorophosphate (BOP) was used as the condensing agent in the reaction of the substituted benzoic acid (1) with (S)-2-(aminomethyl)-N-cyclopropylmethylpyrrolidine (2) (Scheme 1). Isolated yields of the final amide product 3 were modest (40%). It is likely that the yields may be improved if the phenolic group is protected during the reaction. The amide 3 was found to be stable over several months and stored at 0 to -20 °C.
Scheme 1.
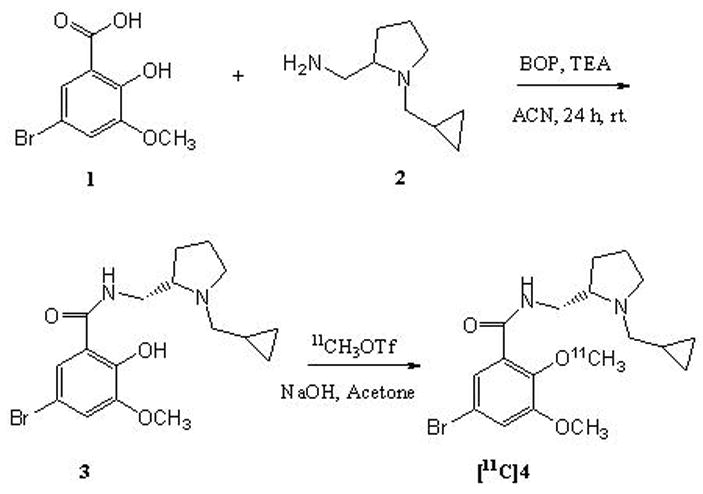
Synthesis of the precursor (3) and radiosynthesis of [11C]4.
2.2. Radiosynthesis
[11C]4 was synthesized with standard methods with minor modifications as previously published for synthesis of [11C]FLB 457.20, 21 The precursor 3 was methylated with [11C]methyl triflate in room temperature, with good incorporation yield 64 ± 11 % (DCY %). The total synthesis time, including the HPLC purification was 28 minutes and specific radioactivity at the time of injection was over 370 GBq/μmol (> 10 000 Ci/mmol)(Figure 2). The radiochemical purity was > 99%. The product [11C]4 was confirmed with a co-elution with the co-injected unlabelled reference (4).
Figure 2.
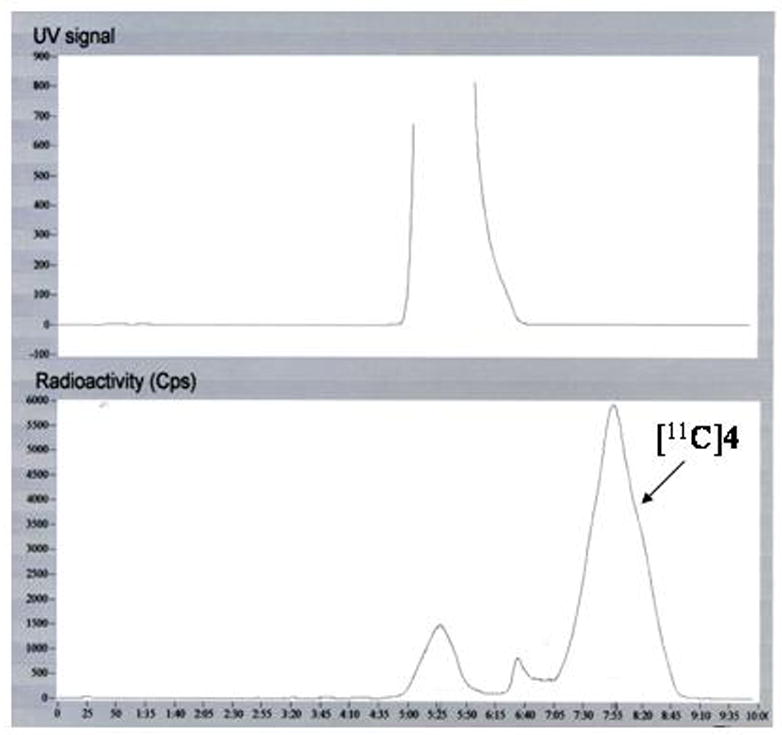
Semipreparative HPLC-chromatogram of [11C]4 purification.
3. Post mortem human brain autoradiography
Autoradiography with [11C]4 in baseline conditions gave high binding in regions with high D2 receptor density, caudate head, putamen and pallidum (Figure 3). [11C]4 bound also with moderate level to thalamus, where the D2-receptor expression is lower. Addition of raclopride (10μM) into the incubation buffer effectively blocked the binding.
Figure 3.
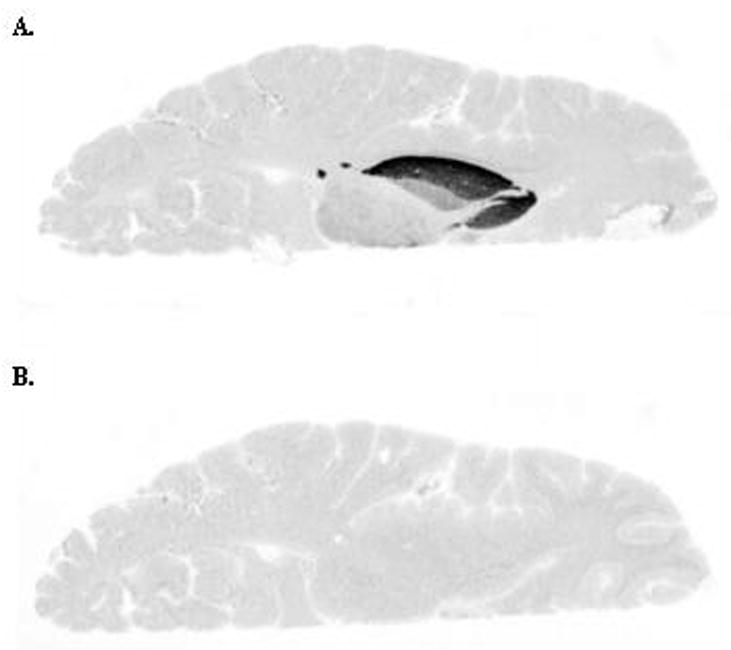
Human brain autoradiograms labelled with [11C]4. A. Baseline conditions. B. Incubation with raclopride (10 μM).
4. PET measurements
Total brain up-take of [11C]4 in cynomolgus monkey was high, reaching 4.4% ID at 7.5 minutes. Binding in D2-receptor rich striatal regions was highest at the end of the PET measurement, without reaching equilibrium during the 93 minutes scanning time (Figures 4 and 5). The highest level of radioactivity was observed in putamen, followed by caudate nucleus and pallidum. Region over cerebellum ratios reached maximum at 84 minutes, giving ratios of 7.61, 6.60 and 5.40, for putamen, caudate nucleus and pallidum, respectively. Extrastriatal regions were clearly visible, indicating sufficient binding affinity also in vivo. The binding was highest in the moderate density receptor region thalamus, followed by cortical regions with low D2-receptor density. Region over cerebellum ratios were at maximum 3.70, 3.24, 2.98, 2.59 and 2.08 for thalamus and frontal, parietal, temporal and occipital cortex, respectively.
Figure 4.
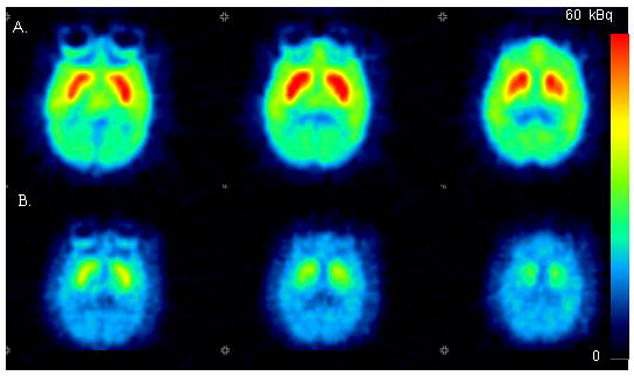
Color coded PET images showing the distribution of radioactivity in the monkey brain after injection of [11C]4. Injected doses were 56 MBq and 50 MBq, respectively. A. Baseline conditions. B. After pretreatment with raclopride (1 mg/kg as tartrate). Summation images from 9th to 93th minute are shown. Anaesthesia was induced and maintained by repeated intramuscular injections of a mixture of ketamine hydrochloride (3.75 mg/kg/h) and xylazine hydrochloride (1.5 mg/kg/h).
Figure 5.
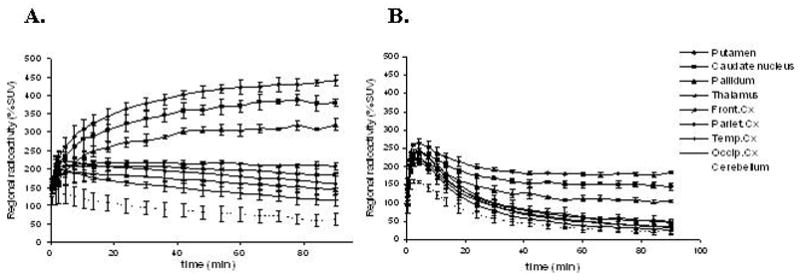
Time activity curves of [11C]4 binding in cynomolgus monkey in baseline conditions (A) and after pre-treatment with raclopride (B).
Specific binding of [11C]4 to D2/D3 receptors was measured after pre-treatment with raclopride (Figure 5). For this preliminary in vivo evaluation, raclopride was chosen for pretreatment agent, because its in vivo behaviour is well characterized. In addition, because it is non-selective dopamine D2 and D3 receptor antagonist, it would clearly demonstrate the amount of non-specific binding in low density D2/D3- receptor brain regions, however without differentiating between these two receptor types. The pretreatment caused substantial reduction in binding in extrastriatal regions, but 40% of the binding in striatal regions remained unblocked. Also 50% of the [11C]4 binding in cerebellum was blocked due to the pre-treatment (Figure 6).
Figure 6.
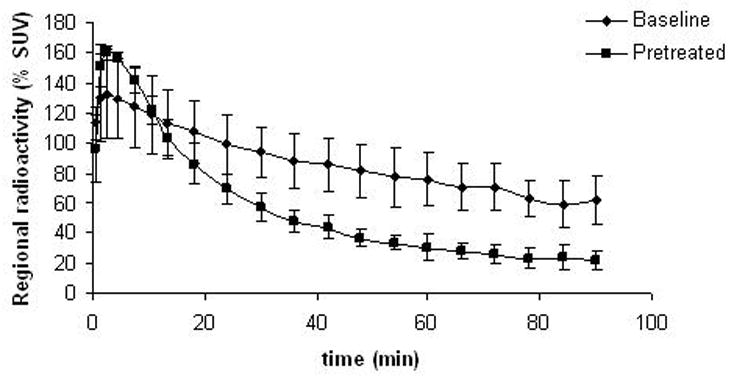
Effect of raclopride pre-treatment (1 mg/kg) on [11C]4 binding in cerebellum.
4.1. Plasma metabolite studies
The HPLC analysis of metabolites in monkey plasma revealed fastest metabolism of [11C]4 during the first 15 minutes (Figure 7). About 60% of the parent compound was left after 45 minutes observation time. Two more polar unidentified radioactive metabolites were found eluting prior the parent compound and more lipophilic radioactive metabolites were not observed (Figure 8). More than 94 % of the injected radioactivity was recovered from the HPLC-column. Radioactivity in pellets after deproteinization with acetonitrile was 8.3 ± 1.9 %.
Figure 7.
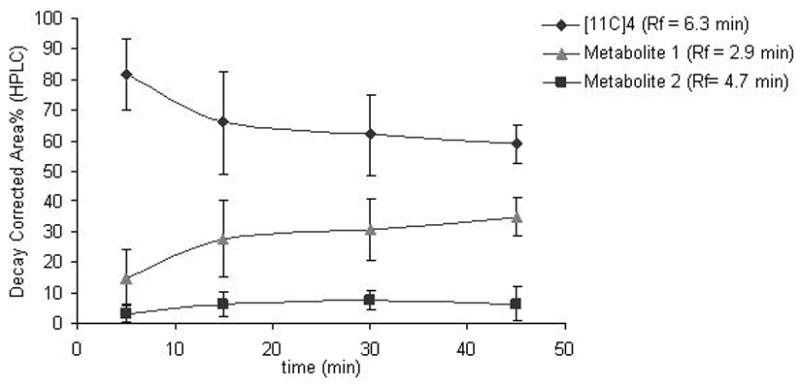
Time course for the metabolism of [11C]4 in monkey plasma (n = 3).
Figure 8.
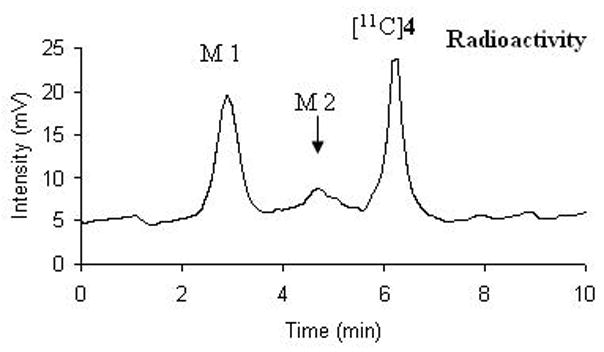
Representative HPLC chromatogram of radioactive metabolites of [11C]4 in monkey plasma 30 minutes after tracer injection.
5. In vitro lipophilicity and plasma protein binding
Apparent partition coefficient of [11C]4 in pH 7.4 was 1.82 ± 0.01. Partition coefficient of [11C]FLB 457 was measured as a control. The measured partition coefficient of [11C]FLB 457 was 1.33 ± 0.01. The result confirms the predicted increase in lipophilicity due to introduction of a cyclopropyl methyl group to the N-position of the pyrrolidine side chain. The free fraction of [11C]4 in plasma in baseline conditions was 32 ± 2% and after pre-treatment 36 ± 1 %, giving confirmation that the raclopride pre-treatment had no significant influence on the free fraction.
6. Discussion
Substituted benzamides containing the N-cyclopropylmethyl pyrrolidine ring has been synthesized previously and found to be stable and bound to dopamine receptor subtypes with high binding affinities.19 These derivatives were synthesized using a coupling reaction between the acid fluorides/anhydrides with (S)-2-(aminomethyl)-N-cyclopropylmethylpyrrolidine (2). For this study, in order to prepare the precursor amide 3, which contains a free phenolic group at the 2-position, we used BOP instead, for assisting in the coupling of the substituted salicylic acid 1 with (S)-2-(aminomethyl)-N-cyclopropylmethylpyrrolidine (2). Product yields were somewhat lower compared to the acid fluoride/anhydride method (40% vs. 70–80%).
The radiolabelled [11C]4 was synthesized via methylation using [11C]methyl triflate with good incorporation yield and with high specific radioactivity (> 10 000 Ci/mmol). For the structural analogue [11C]FLB 457 the co-injected mass of 0.5 μg has been estimated to cause 5 % occupancy in extrastriatal D2/D3 binding sites in humans.22 In our study, in baseline scans, carrier masses of [11C]4 were 0.005 μg and 0.001 μg for cynomolgus monkeys weighting 2.4 and 4.2 kg, respectively. The low injected mass is not presumed to significantly influence the [11C]4 binding even in the low density regions. The binding of [11C]4 in extrastriatal regions, in thalamus and cortical regions was clearly visible and it exhibited relatively high binding in cerebellum, from which about 50% was blocked after pretreatment with raclopride. This result is in accordance with recent findings using [11C]FLB 457 in rats, where 60% of the total cerebellar binding corresponded to specific binding.23 Due to the relatively high level of specific binding in cerebellum, the level of specific binding in extrastriatal regions is underestimated, when cerebellum is used as reference region.
In striatal regions [11C]4 exhibited slow kinetics, which has been also a disadvantage of other high affinity tracers, such as [11C]FLB 457 and [11C]fallypride.5, 24 In addition to the high binding affinity of [11C]4, the slow wash-out may be partly a result of the relatively high lipophilicity (logD7.4 ([11C]4) = 1.82 ± 0.01) caused by the additional cyclopropylmethyl group. The lipophilicity of [11C]4 may be even underestimated in our experimental setup, because the measured partition coefficient of the reference compound [11C]FLB 457 was lower to what has been published by Wilson et al. with the same procedure ([11C]FLB 457 logD7.4 = 1.71 ± 0.02).25
The pretreatment with raclopride did not completely block binding of [11C]4 in striatal regions, only 60% reduction in binding was observed. In post-mortem autoradiography, binding of [11C]4 was totally prevented by 10 mM raclopride, which may be explained by that in autoradiography, the conditions for washing and binding competition are more harsh than in vivo. The preliminary results of the corresponding 18F-labelled cyclopropyl analogue of fallypride exhibited high non-specific binding in a rhesus monkey.19 In addition, the compound showed rapid accumulation of metabolites of the radiotracer. The HPLC-analysis of [11C]4 and its radioactive metabolites in monkey plasma, did not reveal any indication on lipophilic radiolabeled metabolites, which may enter the brain.
Compound 4 has a higher selectivity to D2-receptors over D3-receptors in vitro than the commonly used D2/D3 receptor antagonists FLB 457 and fallypride. Moderate densities of D3 receptors are expressed in thalamus and there are estimates that even one third of the receptor population in pallidum corresponds to D3 receptors.14 The compound [11C]4 did not exhibit any significantly lower uptake in these regions, showing no clear evidence for higher D2-selectivity over D3-receptors in vivo. For studying D2 selectivity of [11C]4 binding, further experiments using D3-selective compounds and comparison to commonly used [11C] labelled D2/D3 receptor ligands are needed.
7. Conclusion
The D2 receptor antagonist [11C]4 exhibited high level of specific binding to brain regions with dopamine D2 receptors in post mortem human autoradiography and in vivo in cynomolgus monkeys. Analysis of radiolabelled metabolites of [11C]4 from monkey plasma revealed that only more polar metabolites were formed, which are unlikely to enter the brain. No clear indication for higher D2 selectivity over D3-receptors of [11C]4 was demonstrated in the present study, but the observed high specific binding in low density D2 regions gives an indication for the high binding affinity also in vivo. In conclusion, [11C]4 is a potential radioligand for studying extrastriatal low density D2 regions, including cerebellum.
8. Experimental
8.1 Chemistry
Commercial reagents and solvents (Aldrich Chemical Company, Milwaukee, WI, USA) of analytical grade were used without further purification. 5-Bromo-2-hydroxy-3-methoxybenzoic acid (1), (S)-2-(aminomethyl)-N-cyclopropylmethylpyrrolidine (2) and the reference compound (S)-5-bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl) methyl]-2,3-dimethoxybenzamide (4) were prepared according to previously described methods.19, 26 Reaction mixtures and extractions were evaporated in a rotavapory evaporator under reduced pressure as appropriate. Analytical and preparative thin layer chromatography (TLC) was performed using Baker-flex silica gel IB-F (Philipsburg, NJ USA) and Alltech DC-Fertigplatten SIL G-200 UV254 plates (Deerfield, IL USA), respectively. Proton nuclear magnetic resonance (NMR, 500 MHz, TMS as internal reference in CDC13) spectra were obtained on a Bruker-Omega 500 spectrometer. Electrospray mass spectra were obtained on a Model 7250 mass spectrometer (Micromass LCT).
All reagents used for radiosynthesis were purchased from commercial suppliers and used without further purification. Acetone and 0.5 M NaOH solution were from Merck Chemicals Ltd. HPLC solvents were HPLC grade from Fisher Scientific. [11C]Methane was produced in a GEMS PETtrace cyclotron by bombardment of a nitrogen gas target containing 10% of H2 with 16 MeV protons (14N(p,α)11C reaction). The target gas was irradiated for 20 minutes with a beam intensity of 35 μA. The synthesis and purification of the radiolabeled compounds was performed in a fully automated methylation system that has been described earlier.20 [11C]Methyl iodide was prepared from [11C]methane by gas-phase iodination. [11C]Methyl triflate was obtained by sweeping [11C]methyl iodide vapor through a glass column containing silver-triflate-impregnated graphitized carbon (Fluka AG) and heated at 150 – 200 °C. 20, 27
[11C]4 was purified in a built-in high performance liquid chromatography (HPLC) system, consisting of a Gilson 234 autoinjector (Middleton, MA, USA), a Gilson 304 piston pump, a Waters μPorasil silica column (300 x 7.8 mm, 10 μm) and a Gilson 118 UV/VIS detector (wavelength 254 nm) in a series with a Geiger Müller (GM) tube for radiation detection. Radiochemical purity of [11C]4 was analyzed on reverse phase HPLC using a Merck-Hitachi L-7100 Pump, equipped with a Waters μBondapak C18 column (300 x 3.9 mm, 10 μm) (Milford, MA, USA) and L-7400 UV-detector, D-7000 interface and Beckman radiodetector (Model 170). The system was controlled by Merck-Hitachi Chromatography Data Station Software D-7000 (version 4.1). Acetonitrile (30%) in 0.01M H3PO4 was used as mobile phase. Specific radioactivity of [11C]4 was analyzed with HPLC, consisting Waters μ-Bondapak C18 column (300 x 3.9 mm, 10 μm), Merck-Hitachi L-6200A intelligent pump and Merck-Hitachi L-4000 UV (254 nm) detector. The mobile phase was acetonitrile in 0.05 M H3PO4 (18/82) and flow rate was 3 mL/min. The product eluted at 12–13 minutes.
8.2. (S)-5-Bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-2-hydroxy-3-methoxybenz-amide (3)
To a solution of 5-bromo-2-hydroxy-3-methoxybenzoic acid, 1 (0.25 g, 1 mmol) in anhydrous acetonitrile (5 mL) was added (S)-2-(aminomethyl)-N-cyclopropylmethylpyrrolidine 2 (0.17g, 1.1 mmol). This mixture was stirred at room temperature and BOP reagent (benzotriazol-1-yloxy-tris(dimethylamino)phosphonium hexafluorophosphate, 0.44 g, 1 mmol) and triethylamine (0.4 mL) was added. The reaction mixture was stirred for 24 hrs. Volatiles were removed in vacuo and the residue was taken in dichloromethane and washed with water and saturated sodium bicarbonate. The organic layer was separated, dried over magnesium sulfate and filtered. The filtrate was concentrated and subsequently purified using preparative TLC (silica gel, dichloromethane: methanol, 9:1) to provide 3 in 40% yield. 1H NMR δ ppm 8.20 (br, 1 H, CONH), 7.4 (d, 1H), 7.0 (d, 1H), 3.8 (s, 3H, OCH3), 3.1-1.5 (m, 11H), 0.30 (5H, c-C3H5). Electrospray Mass spectra (m/z, %) 383, 385 ([M+H]+, 100%).
8.3. (S)-5-Bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-2-[11C]methoxy-3-methoxy-benzamide, [11C]4
(S)-5-Bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-2-hydroxy-3-methoxybenzamide (3, 0.5 mg, 1.3 μmol) was dissolved in acetone (400 μL) and 0.5 M NaOH (6 μL, 3 μmol) was added. The mixture was bubbled with [11C]methyl triflate at room temperature. The product was purified with the semi-preparative HPLC system with CH2Cl2/MeOH/TEA (95/5/0.5, pH 8.0) as eluent with flow 2 mL/min from 0 to 4 minutes and 4 mL/min after that. Retention time of the product was 6.3 min. The collected fraction, containing the purified product, was evaporated to dryness and formulated in 7 mL of phosphate buffer (pH 7.4) and 3 mL of 30% EtOH in propylene glycol (v/v). Incorporation yield was 64 ± 11 %, with the total synthesis time of 28 minutes. Specific radioactivity at the time of injection was > 370 GBq/μmol (> 10 000 Ci/mmol). Purity was analyzed with the analytical HPLC system with flow 3 mL/min. The product was identified with a co-injection of the reference. Retention time of the product was 4.18 min. Radiochemical purity was > 99%.
9. Post mortem human brain autoradiography
Horizontal whole hemisphere post mortem human brain sections of 100 μm thickness from the level of striatum were incubated for 20 minutes at room temperature with [11C]4 (approx. 30 MBq) in Tris-buffer (50 mM, pH 7.4), containing 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, Pargylin (10 μM) and 0.1% acetic acid. The D2/D3 antagonist raclopride (10 μM) was included in the incubation medium to determine the level of non-specific binding. After incubation, the sections were washed with ice-cold Tris buffer (50 mM, pH 7.4) for 2 x 2 min, briefly dipped into ice-cold distilled water and dried with warm air flow. Phosphor imager plates (BAS-IP SR 2025, Fuji Photo Film, Japan) were used for detection of the radioactivity distribution. The plates were exposed for 20 minutes, scanned and analyzed with Fuji BAS-5000 image reader, running with Fuji Film Multi Gauge software.
10. PET measurements
10.1. PET system
The radioactivity distribution was measured using a Siemens ECAT EXACT HR PET camera system (CTI/Siemens, Knoxville, TN) in three-dimensional mode, which measures radioactivity in 47 sections with a separation of 3.1 mm and a transversal resolution of about 3.8 mm FWHM (Full Width Half Maximum). The attenuation correction of the data was obtained with three rotating 68Ge rod sources. Raw PET data were then reconstructed using the standard filtered back projection consisting of the following reconstruction parameters: 2 mm Hanning Filter, scatter correction, a zoom factor of 2.17 and a 128 x 128 matrix seize.28
10.2. PET experimental procedure
PET measurements were performed in two female cynomolgus monkeys (Macaca fascicularis), weighting 2.4 and 4.2 kg. The monkeys were supplied by Astrid Fagraeus Laboratory, Swedish Institute for Infectious Disease Control (SMI), Solna, Sweden. The study was approved by the Animal Research Ethical Committee of the Northern Stockholm Region. Anaesthesia was induced and maintained by repeated intramuscular injections of a mixture of ketamine hydrochloride (3.75 mg/kg/h, Ketalar®, Pfizer) and xylazine hydrochloride (1.5 mg/kg/h Rompun®, Vet. Bayer) for the duration of each measurement. A head fixation system was used to secure a fixed position of the monkey head during the PET experiments.29 Body temperature was maintained by Bair Hugger – Model 505 (Arizant Healthcare Inc., MN) and monitored by a rectal thermometer (Precision Thermometer, Harvard Apparatus, MA). Cardiac and respiratory rates were monitored continuously throughout the experiment (VetGard monitor, Veterinary Marketing Systems, Lincoln, NE).
Both monkeys participated in one baseline and one pre-treatment measurement, respectively. In the first measurement, 56.2 ± 0.2 MBq of [11C]4 was injected as a bolus during 5 sec into a sural vein and radioactivity in the brain was measured according to a pre-programmed sequence of frames up to 93 minutes after the injection of the tracer. Injected mass in the baseline experiments was 0.005 μg and 0.001 μg, respectively. In the second measurement 1.0 mg/kg of raclopride-tartrate was injected intravenously to the same monkey 35 minutes before injection of [11C]4 (52.5 ± 2.5 MBq, injected mass 0.01 μg and 0.002 μg) and the radioactivity in the brain was measured as previously described.
10.3. Quantitative image analysis
PET summation images representing radioactivity measured from 9 to 93 minutes after an intravenous injection of [11C]4 were generated. The baseline PET images were transformed into a standard anatomical space using the monkey version of the Human Brain Atlas developed at Karolinska Institute.30 The transformation matrix generated on this image was applied to all the frames from the corresponding and the consecutively performed pretreatment PET study.
A standard template of ROIs was generated on an average monkey MRI scan and applied to each PET study. ROIs were delineated for the putamen, caudate nucleus, pallidum, thalamus, frontal, occipital, parietal and temporal cortex, cerebellum and whole brain. The ROI of the cerebellum excluded the vermis and white matter.
All calculations were based on the assumption that radioactivity in brain represents unchanged radioligand. The fraction of [11C]4 in whole brain relative to the total amount of radioligand injected was calculated and plotted versus time after injection. The radioactivity concentration in the ROI for the whole brain was multiplied with the whole brain ROI volume (~ 65 mL), divided by the radioactivity injected and multiplied by 100 to obtain the percentage.
Regional radioactivity was normalized to injected activity and body weight by use of % of standard uptake value (%SUV = (% injection dose/cm3 brain) x body weight (g)). The cerebellum was used as a reference region for non-displaceable [11C]4 binding. The radioactivity in the cerebellum was accordingly used as an approximate for free and non-specifically bound radioligand concentration in brain. The time curve for specific [11C]4 binding to D2 receptors in high-density brain regions was defined as the difference between the total radioactivity concentration in a ROI and the cerebellum.
10.4. Plasma metabolite studies
For the analysis of radioactive metabolites in monkey plasma, blood samples of 2.0 mL were obtained from the femoral vein of a monkey 4, 15, 30 and 45 minutes after injection of the tracer. Fractions of radioactivity in the monkey plasma that corresponded to unchanged [11C]4 and radioactive metabolites were determined using a modification of a gradient method described by Halldin et al. 1995.31 Shortly, the blood samples were centrifuged at 2000 x g for 2 min, and the plasma (0.5 mL) was mixed with acetonitrile (0.7 mL) and centrifuged at 2000 x g for 2 min. The supernatant of the acetonitrile plasma mixture (1.1 mL) was injected to μBondapak C18 (300 x 7.8 mm, 10 μm) HPLC column and analyzed with a gradient of acetonitrile (A) and phosphoric acid (0.01M) (B) with a program: 0 min (A/B) 10:90, 5 min (A/B) 80:20, 6.5 min (A/B) 10:90. Flow was 6 ml/min. Radioactivity of the injected supernatant, protein pellet, the residual in the HPLC syringe and the collected radioactivity from the HPLC were measured with a NaI well counter for calculation of total recovery of the metabolite analysis.
11. In vitro lipophilicity and plasma protein binding
Lipophilicity of [11C]4 in pH 7.4 (logD7.4) was determined with a shake-flask method.25 Partition coefficient of [11C]FLB 457 was measured as a standard. In short, the tracer solution (40 μl) was added to a separation funnel, containing 1-octanol (10 mL) and phosphate buffer (10 ml, pH 7.4). The flask was shaken 3 minutes and the aqueous layer discarded. Another portion of phosphate buffer (10 mL) was added and the procedure was repeated. Four portions (2 mL each) of the 1-octanol layer were pipetted to four test tubes and phosphate buffer (2 mL to each) was added. The tubes were vortexed for 2 minutes and centrifuged 4 min in 2000 x g. Samples (0.5 mL) were pipetted from the both layers for counting. The partition coefficient (D) was calculated using Eq.1:
| Equation 1 |
Plasma protein binding of [11C]4 was measured in duplicate in baseline conditions and after pre-treatment with raclopride. Monkey plasma (500 μL) or 0.9% NaCl-solution as a control, was mixed with [11C]4 solution (100 μL, approx. 10 MBq) and incubated in room temperature for 10 minutes. Samples (20 μL) from each incubation mixture were measured with a well-counter. After the incubation, 100 μL portions of the incubation mixtures were pipetted into ultra-filtration tubes (Millipore Centrifree YM-30) and centrifuged for 10 min in 2000 rpm and 5 min in 3000 rpm (Sigma-202, Axel Johnson Lab System). Samples (20 μL) from each filtrate were pipetted for counting. The results were corrected for the membrane binding as measured with the control samples.
Acknowledgments
We thank Phong Truong, Siv Eriksson and Dr. Baogang Xue for technical assistance. A part of this work was supported by grant from Academy of Finland (decision number: 115385) and grant number R01EB006110 from the National Institute of Biomedical Imaging and Bioengineering (USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halldin C, Farde L, Högberg T, Mohell N, Hall H, Suhara T, Karlsson P, Nakashima Y, Swahn CG. J Nucl Med. 1995;36:1275. [PubMed] [Google Scholar]
- 2.Olsson H, Halldin C, Swahn CG, Farde L. J Cereb Blood Flow Metab. 1999;19:1164. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Okauchi T, Suhara T, Maeda J, Kawabe K, Obayashi S, Suzuki K. Synapse. 2001;41:87. doi: 10.1002/syn.1063. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narakyanan TK, Satter M, Mantil J. Synapse. 2002;46:170. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee J, Shi B, Christian BT, Chattopadhyay S, Narayanan TK. Bioorg Med Chem. 2004;12:95. doi: 10.1016/j.bmc.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Antonini A, Scwarz J, Oertel WH, Pogarell O, Leenders KL. Mov Disord. 1997;12:33. doi: 10.1002/mds.870120107. [DOI] [PubMed] [Google Scholar]
- 7.Thobois S, Jahanshahi M, Pinto S, Frackowiak R, Limousin-Dowsey P. Neuroimage. 2004;23:1. doi: 10.1016/j.neuroimage.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Hirvonen J, van Erp TGM, Huttunen J, Någren K, Huttunen M, Aalto S, Lönnqvist J, Kaprio J, Cannon TD, Hietala J. Psychiatry Res Neuroimaging. 2006;146:13. doi: 10.1016/j.pscychresns.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Talvik M, Nordström AL, Okubo Y, Olsson H, Borg J, Halldin C, Farde L. Psychiatry Res Neuroimaging. 2006;148:165. doi: 10.1016/j.pscychresns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Mayer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NPLG, Wilson AA, Houle S. Am J Psychiatry. 2006;163:1594. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RM, Whetsell WO, Ansari MS, Votaw JR, de Paulis T, Clanton JA, Schmidt DE, Mason NS, Manning RG. Brain Res. 1993;609:237. doi: 10.1016/0006-8993(93)90878-q. [DOI] [PubMed] [Google Scholar]
- 12.Schotte A, Janssen PFM, Gommeren W, Luyten WHML, van Gompel P, Lesage AS, de Loore K, Leysen JE. Psychopharmacol. 1996;124:57. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 13.Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Synapse. 2006;60:485. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 14.Seeman P, Wilson A, Gmeiner P, Kapur S. Synapse. 2006;60:205. doi: 10.1002/syn.20298. [DOI] [PubMed] [Google Scholar]
- 15.Rabiner EA, Raymond R, Diwan M, McCormick P, Wilson AA, Nobrega J. J Nuc Med. 2007;48(Suppl 2):113P. [Google Scholar]
- 16.Vogel M, Busse S, Freyberger HJ, Grabe HJ. Medical Hypothese. 2006;67:354. doi: 10.1016/j.mehy.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Joyce JN, Millan MJ. Drug discov today. 2005;10:917. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- 18.Heidbreder CA, Gardner EL, Zheng-Xiong X, Panayotis KT, Mugnaini M, Hagan JJ, Ashby CR., Jr Brain Res Reviews. 2005;49:77. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z-Y, Mukherjee J Med Chem Res. 1999;9:1. [Google Scholar]
- 20.Sandell J, Langer O, Larsen P, Dollé F, Vaufrey F, Demphel S, Crouzel C, Halldin C. J Labell Compd Radiopharm. 2000;43:331. [Google Scholar]
- 21.Lundkvist C, Sandell J, Någren K, Pike VW, Halldin C. J Labell Compd Radiopharm. 1998;41:545. [Google Scholar]
- 22.Olsson H, Halldin C, Farde L. Neuroimage. 2004;22:794. doi: 10.1016/j.neuroimage.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Asselin MC, Montgomery AJ, Grasby PM, Hume SP. J Cereb Blood Flow Metabl. 2007;27:378. doi: 10.1038/sj.jcbfm.9600340. [DOI] [PubMed] [Google Scholar]
- 24.Olsson H, Farde L. Neuroimage. 2001;14:936. doi: 10.1006/nimg.2001.0879. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AA, Jin L, Garcia A, DaSilva JN, Houle S. Appl Radiat Isotop. 2001;54:203. doi: 10.1016/s0969-8043(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 26.Hogberg T, de Paulis T, Johansson L, Kumar Y, Hall H, Ogren SO. J Med Chem. 1990;33:2305. doi: 10.1021/jm00170a040. [DOI] [PubMed] [Google Scholar]
- 27.Jewett DM. Appl Radiat Isot. 1992;43:1383. doi: 10.1016/0883-2889(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.Wienhard K, Dahlbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, Heiss WDT. J Comput Assist Tomogr. 1994;18:110. [PubMed] [Google Scholar]
- 29.Karlsson P, Farde L, Halldin C, Swahn C, Sedvall G, Foged C, Hansen KT, Skrumsager B. Psychopharmacology (Berl) 1993;113:149. doi: 10.1007/BF02245691. [DOI] [PubMed] [Google Scholar]
- 30.Roland PE, Zilles K. Trends Neurosci. 1994;17:458. doi: 10.1016/0166-2236(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 31.Halldin C, Swan C-G, Farde L, Sedvall G. In: PET for drug development and evaluation. Comar D, editor. Kluwer; Dordrecht: 1995. pp. 55–65. [Google Scholar]


